The Importance of CAP Accreditation in Diagnostic Testing: 3billion’s Journey
3billion obtained CAP accreditation in December 2021 with an excellent score. Thanks to team members who worked day and night to prepare for the inspection, we wrapped up 2021 with good news. Once again, a big shoutout to everyone involved.

Wherever diagnostic testing is offered, the words CAP-accredited or CLIA-certified naturally come up. Overseas doctors and researchers who are interested in our testing often ask first whether 3billion has obtained any such accreditation. Perhaps this is because CAP accreditation serves as the first criterion to determine whether a laboratory is trustworthy.
What is CAP?
Abbreviation for College of American Pathologists, CAP was founded in 1961 in the United States for laboratory quality control and is the world’s most prestigious accreditation body.
Since CAP accreditation is known as the gold standard for laboratory quality control worldwide, if a laboratory is CAP-accredited, it can be understood that the laboratory is operating with quality control standards that are strict enough to conduct testing for clinical diagnostic purposes.
What is Quality Control in diagnostic testing?
I think there may be some who are unsure about the term Quality Control. If anyone asks, “How does your laboratory perform quality control?” you can think of it as the same question as “What kind of management system do you have to increase the reliability of the results produced in your laboratory?”
Since through diagnostic testing, a patient’s symptoms are translated into objective numerical values or results, it is natural that stringent quality control is needed in a diagnostic testing laboratory.
For CAP accreditation, all internal procedures that are related to the testing must be documented in a Standard Operating Procedure (SOP). Based on this, the CAP inspector evaluates whether there are any issues in various aspects of the experimental procedure. Documentation of all laboratory procedures means that all experimental or analytical procedures are structured and established. In other words, this indicates that all processes in the laboratory maintain the same level of results regardless of when or who carries them out and a high level of 1) accuracy, 2) sensitivity, 3) reproducibility, and 4) stability of results.
In addition, the accuracy of the results produced in the laboratory is an important evaluation of the CAP accreditation process, but it is also very important to evaluate whether there is sufficient stability in terms of handling patient information. Therefore, how systematically the storage and access of patient data is managed, whether the procedure for storing the patient’s data is appropriate, and whether the consent and guidance procedure are accurate are also subject to evaluation.
For reference, CLIA certification is often referred to alongside CAP accreditation. CLIA is focused on US federal law/regulation, and CAP includes all the items required by CLIA, as well as standards for overall laboratory management and operations. Thus, 3billion focused on obtaining CAP accreditation last year.
Procedures for CAP accreditation
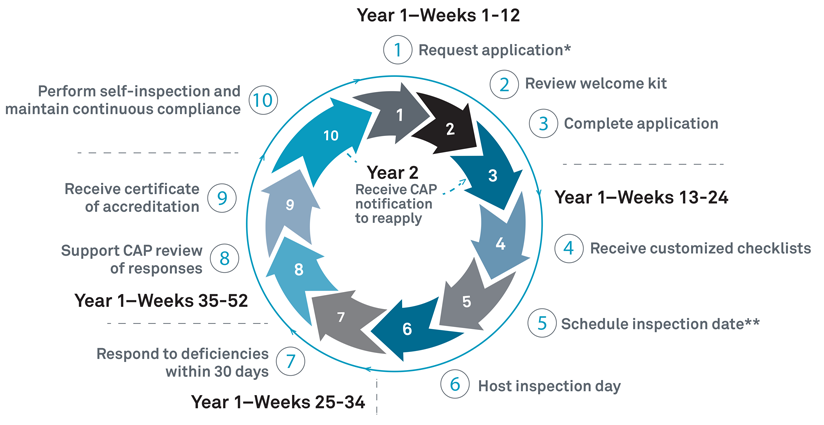
Accreditation Process and Timelines.
reference: https://www.cap.org/laboratory-improvement/accreditation/accreditation-process
The diagram above shows the CAP accreditation process provided on the CAP official website (cap.org). After applying for accreditation, receiving a checklist suitable for the particular laboratory, preparing to pass the checklist, confirming the inspection date, undergoing the inspection, and supplementing the deficiencies received from the inspector after the inspection, you will finally obtain the CAP accreditation you have been waiting for. In fact, a lot of our resources were dedicated to the process of supplementing and documenting our procedures in accordance with the checklists.
Once we receive CAP accreditation, will it last forever? Of course not. An on-site inspection and self-inspection are performed biennially to assess whether the laboratory is still well maintained in accordance with CAP accreditation standards. The on-site inspection and self-inspection are conducted every other year, so there is an assessment every year.
There are moments when I suddenly realize that the work I do actually affects the lives of patients. At such times, regular daily tasks suddenly feel different and precious. As aforementioned, diagnostic testing is a medical action that involves translating the patient’s condition into objective numerical values. Thus, 3billion’s rigorous management of testing procedures following CAP regulations is an aspect that cannot be overlooked as it can affect the lives of patients.
Get exclusive rare disease updates
from 3billion.

Sookjin Lee
Expert in integrating cutting-edge genomic healthcare technologies with market needs. With 15+ years of experience, driving impactful changes in global healthcare.






