Comparing reanalysis policies of 5 rare disease diagnostics companies
Although many are struggling, the initial diagnosis rate of rare diseases remains low. It is because there is still insufficient information and the symptoms are very diverse. Therefore, diagnosis of rare diseases should not be limited to a single analysis, so the importance of reanalysis is inevitably greater.
In this article, we will take a look at the reanalysis policies that each rare disease diagnosis company operates.
What is Reanalysis?
In one of our previous blog posts, we looked into ‘why reanalysis is important?’ and ‘the difference between reanalysis and reclassification.’ Genomic data is reanalyzed in the below circumstances:
- When genetic variant information related to a disease is newly added
- When the patient discovers new symptoms or the symptoms change
- When the analyzing company’s diagnostic algorithm changes

Curious How We Boost Diagnostic Accuracy?
Get the newsletter every month and discover the breakthroughs with the latest reanalysis report.
What are the criteria for selecting comparison targets?
- Companies that directly receive patient samples and provide a diagnostic report
- Companies that receive CAP/CLIA accreditation
- Companies offering additional services other than WES
- Companies conducting global business
Looking at a glance
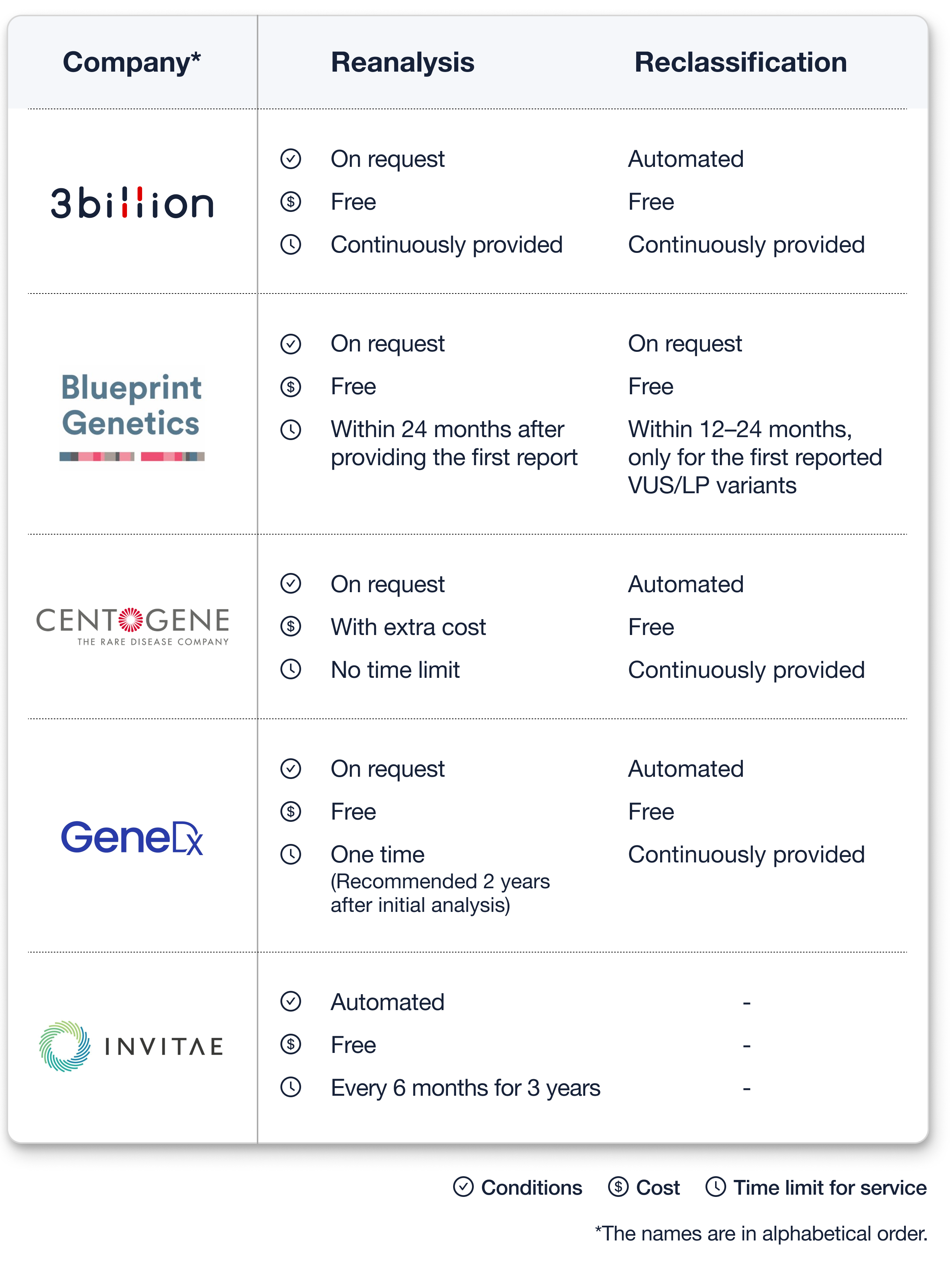
- Blueprint Genetics
Reevaluation can be requested for 12–24 months from the time the first result is provided, and its scope is limited to VUS and LP mutations included in the first report.
Reanalysis is a case of reanalyzing the entire WES data and can be requested within 24 months from the first result. - Centogene
Reevaluation and reclassification are provided in the first filtered mutation level at no extra cost.
Reanalysis is performed on the sample that received uncertain or negative results in the initially provided results, and a separate fee is charged. - GeneDx
They do not discriminate between reevaluation and reanalysis. For reanalysis, they provide only a one-time service; hence, it is recommended to request it after 2 years of receiving the first result. - Invitae
Reevaluation is automatically performed continuously, and reanalysis is performed automatically every 6 months for 3 years and additional analysis results are provided. - 3billion
The unique part of 3billion is that the database on which the diagnosis is based is updated daily to make the diagnosis with the most up-to-date information.
Reevaluation is performed routinely via an automatically updated database, and a new diagnosis is immediately delivered to the medical staff.
Reanalysis is performed upon request by the medical staff when the patient’s symptoms change or additional family history is obtained. WES is continuously provided until a patient is diagnosed, without extra costs or frequency/period limitations.
There is a standardized methodology in the diagnostic process of rare diseases, but there is no single way to find the answer.
It is because there are many types of diseases, and even patients with the same disease show a diverse range of symptoms. Thus, it is difficult for a single medical professional or a single company to find all the answers. Hence, it is important that the genetic diagnostic service providers for rare diseases to share their knowledge and experience (variant registration in ClinVar, conference presentations, webinars, white papers, etc.) and work toward the common goal of providing diagnostics to more patients rather than viewing each other as competitors.
Get exclusive rare disease updates
from 3billion.

Sookjin Lee
Expert in integrating cutting-edge genomic healthcare technologies with market needs. With 15+ years of experience, driving impactful changes in global healthcare.







