What are the ACMG Standards and Guidelines and how do they work?
Recently, Next Generation Sequencing (NGS) technology such as Whole Exome Sequencing (WES) and Whole Genome Sequencing (WGS) has been increasingly used for the diagnosis of patients with rare diseases. By WES, about 80,000 to 100,000 genetic variants can be found per person. ACMG Standards and Guidelines are followed for variant interpretation and prioritization to ultimately identify one or two disease-causing variants.
Let’s learn about the ACMG guidelines and how they are applied, along with real-life examples of variant interpretation.
What are the ACMG Guidelines?
The ACMG Guidelines are internationally accepted guidelines for the interpretation of variants. Updated in 2015, the current version has become the standard for terminology and methods as the scope of clinical genetic testing has gradually diversified with the development of sequencing technology.
The ACMG Guidelines were established by clinicians and clinical lab directors who are experts in clinical genetics and part of the American College of Medical Genetics and Genomics (ACMG), Association for Molecular Pathology (AMP), and College of American Pathologists (CAP).
What are the specific criteria of the ACMG guidelines?
There are 28 criteria in the ACMG guidelines. During variant interpretation, variants are classified into five tiers: Pathogenic (P), Likely pathogenic (LP), Uncertain significance (VUS), Likely benign (LB), and Benign (B), depending on the applied criteria. 28 criteria can be classified by the weight and type of evidence indicated by each criterion.
- Classification by evidence weight
The 28 criteria can be classified into two: 16 Pathogenic criteria and 12 Benign criteria. Depending on the weight of evidence, the pathogenic criteria are divided into very strong (PVS1), strong (PS1–4), moderate (PM1–6), supporting (PP1–5); the benign criteria are divided into stand-alone (BA1), strong (BS1–4), supporting (BP1–7). Criteria within the same category are given the same weight. - Classification by evidence type
The 28 criteria can be classified into 8 types: population data, computational data, functional data, segregation data, de novo data, allelic data, other database, and other data, depending on the source of evidence. You can check the details of each criteria in Figure 1 and the ACMG guidelines.
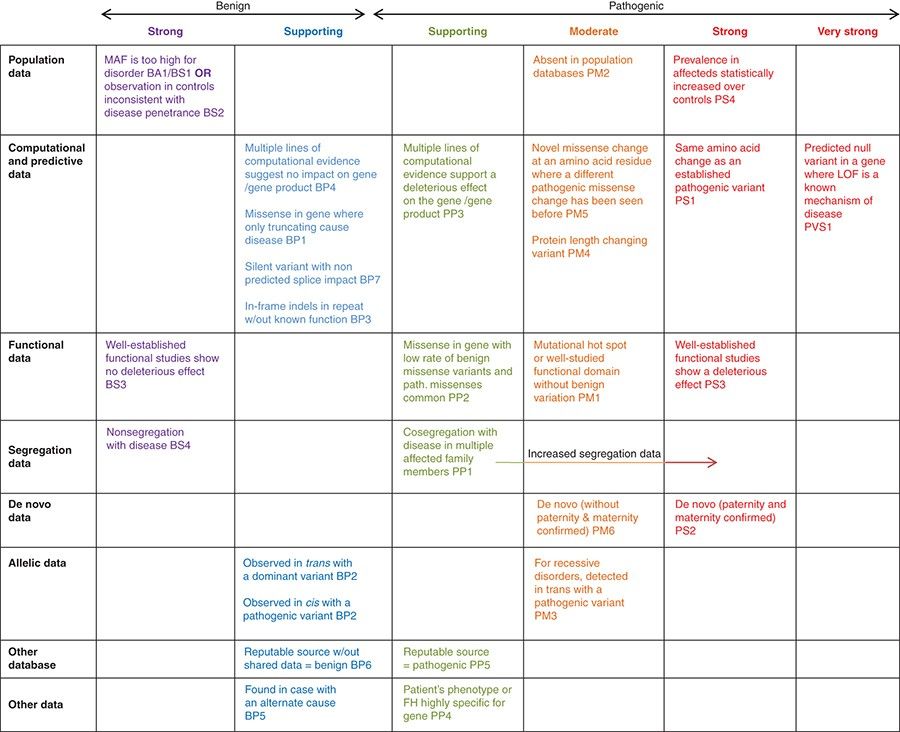
Application of ACMG Guidelines
Let’s take an example of how criteria are assigned with an actual genetic variant.
12-49441847-CAT-C
KMT2D(NM_003482.4)
c.4135_4136del(p.Met1379ValfsTer52)
*The nomenclature follows the HGVS Variant Nomenclature Guidelines. For more information on the nomenclature and variant interpretation, read the other post, “How to read variant information in the rare disease test reports(HGVS nomenclature).”
This is a variant in which bases from 4135 to 4136 of the KMT2D gene have been deleted. The following three criteria were applied according to the evidence collected on the variant.
- PVS1 (null, frameshift)
The PVS1 criteria was applied because it is expected that a frameshift mutation would occur due to the deletion of the bases, causing a loss of normal protein function. - PS2 (case-level data)
The PS2 criteria was applied based on case-level data proving that the mutation is a de novo mutation. - PM2 (absent in normal)
Since the variant was not found in the 100,000-person general population genome database, the rarity of the mutation was recognized and the PM2 criteria was applied.
According to the ACMG’s rules for combining criteria to classify sequence variants, 1 very strong pathogenic criteria, 1 strong and 1 moderate pathogenic criteria were applied and finally classified as a pathogenic variant. In this way, the ACMG guidelines help guide decisions by taking into account both known research results and information about each variant.
Limitation and Future Direction
Even though many clinical labs started using common terms and methods following the ACMG guidelines, resolving many potential discordant results of variant interpretation, it is still not possible to standardize all interpretation results from all labs. This is because results still vary by how and to what extent the criteria are applied, which can lead to confusion when making diagnosis and treatment decisions.
In order to solve this problem and completely standardize the interpretation of variants, it is important to collect and continuously share the results and evidence drawn by institutions and curate each variant accordingly. Clingen Working Groups are striving to modify and update variant classifications specific to disease and gene groups to aid accurate variant evaluation, and Clinvar regularly reports variant reclassifications.
By continuously supplementing variant interpretation guidelines and minimizing the gaps between clinical labs by sharing knowledge and data, we will be able to deliver the most up-to-date and accurate diagnostic results to patients with rare diseases around the world.
The article for 2024 is also ready!

Curious How We Boost Diagnostic Accuracy?
Sign up on our newsletter to discover the breakthroughs with our latest reanalysis report.
Get exclusive rare disease updates
from 3billion.

Sookjin Lee
Expert in integrating cutting-edge genomic healthcare technologies with market needs. With 15+ years of experience, driving impactful changes in global healthcare.







