Why quality control (QC) is performed for NGS testing
For rare disease diagnostic testing, a sample is first collected from the patient. From the sample, genomic DNA is extracted and used for library preparation. Then, whole exome sequencing (WES) is performed. The data obtained through sequencing are processed to identify suspicious variants consistent with the patient’s symptoms, after which medical geneticists make a diagnosis.
Library preparation for whole exome sequencing is a very long process that takes more than 24 hours.
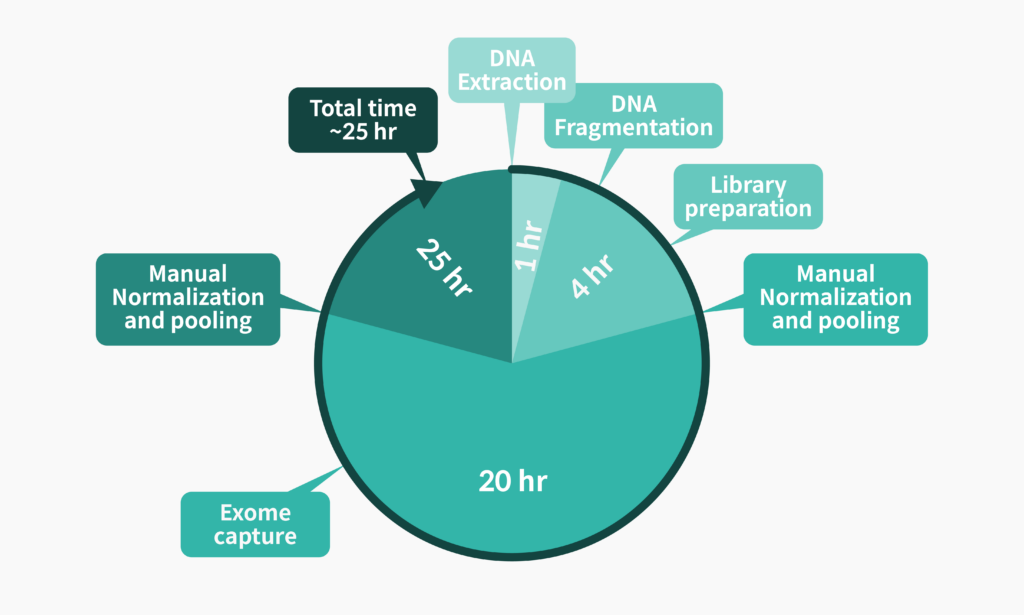
The patient’s sample is often provided in the form of blood or buccal swab, but the amount of genomic DNA that is extracted may not always be sufficient for whole exome sequencing. This is why researchers are very cautious with genomic DNA and conduct testing with the mindset, “This is the first and last chance.”
Notably, 3billion’s molecular genetic laboratory has been accredited by the College of American Pathologists (CAP) and performs world-class quality control to ensure that the experiment is conducted properly every step of the way. The process is as follows.
First, there are two main parts of quality control for WES.
- DNA Quality Control (DNA QC)
- Library Quality Control (Library QC)
DNA Quality Control (DNA QC)
DNA QC is the process of evaluating the quantity, purity, and intactness of the genomic DNA.
First, once DNA is extracted from the patient’s sample (blood or buccal), the DNA is checked to ensure it has been extracted purely without the contamination of proteins or organic solvents (Figure 1).
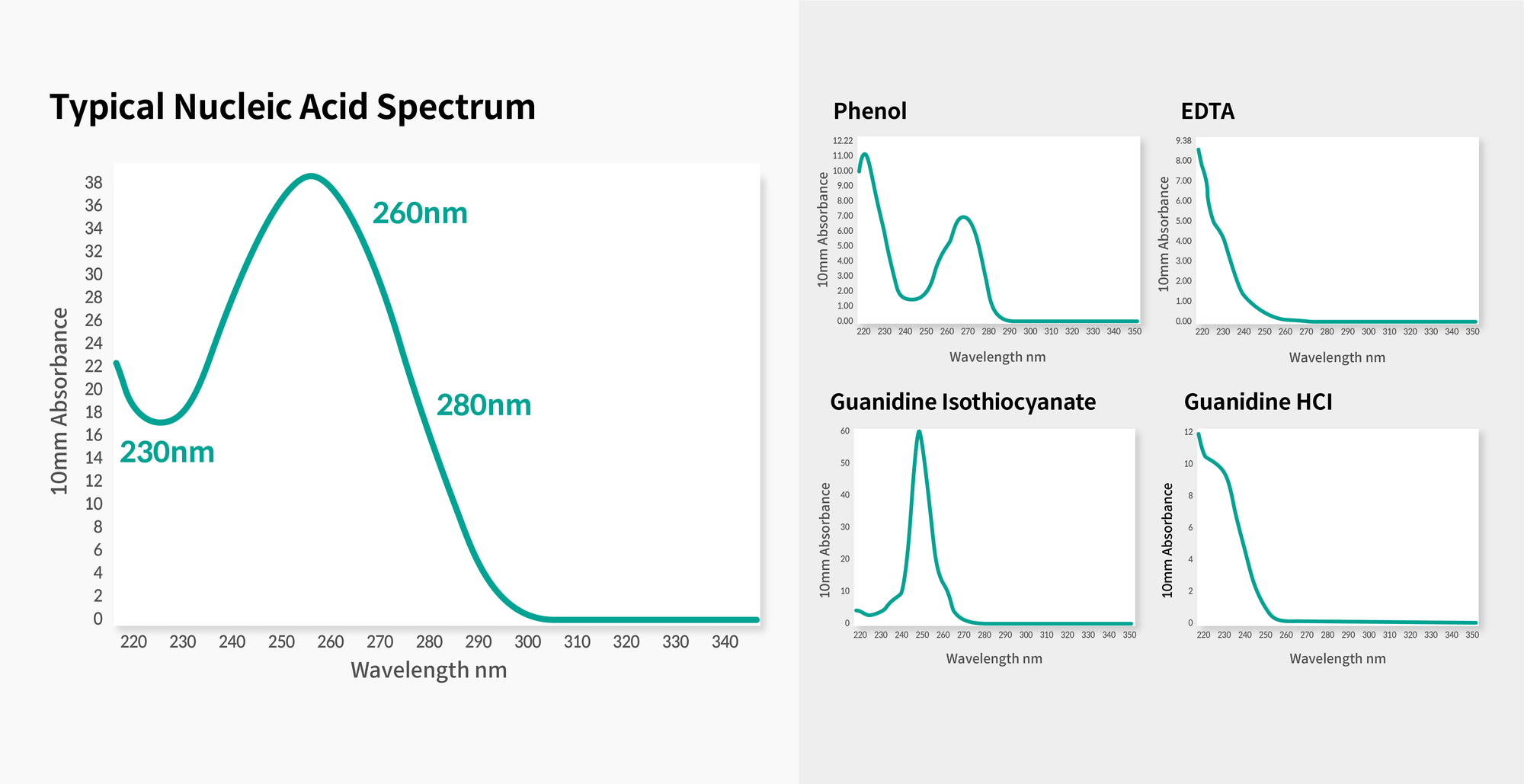
Figure 1. Result of NanoDrop. Examples of high-purity genomic DNA (left) and various possible contaminants (right)
(Reference: https://1ofdmq2n8tc36m6i46scovo2e-wpengine.netdna-ssl.com/wp-content/uploads/2018/10/User-SOP-Genomic-DNA-Sample-QC_v5.pdf)
The DNA is also checked to ensure it is intact (Figure 2). This is because genomic DNA is fragmented into sizes suitable during library preparation, so if the DNA is already fragmented, it will be cut into even smaller fragments, which would be unsuitable and eventually just discarded.

Figure 2. Result of Gel electrophoresis. Although DNA sample 2 has a higher concentration than DNA sample 1, QC fails because an intact band is not seen on the gel.
Finally, to make up for losses that inevitably occur along the test procedures, a DNA binding dye is used to measure the exact quantity of DNA needed and thus prepare a sufficient amount of DNA.
After passing such quality checks, the prepared DNA goes on to the library production stage.
Library Quality Control (Library QC)
In WES, a library refers to the genomic DNA fragment pool of a certain individual. These genomic DNA fragments have an index adapter, which is an essential oligonucleotide with a specific sequence needed for sequencers to recognize the DNA fragments.
Therefore, library QC is the process of checking whether the intact genomic DNA has been cut into DNA fragments of a size recognized by the sequencer and whether the adapters are ligated to the fragments.
First, the appropriate amount of genomic DNA is fragmented into small pieces by a mechanical method in the laboratory, after which the size and amount of the fragments are checked by an automated electrophoresis device.
After attaching adapters to the fragmented DNA, polymerase chain reaction (PCR) is performed to ensure that the library contains a sufficient amount for sequencing. Then, once again, the automated electrophoresis device is used to check the exact size and approximate amount of the library (Figure 3).
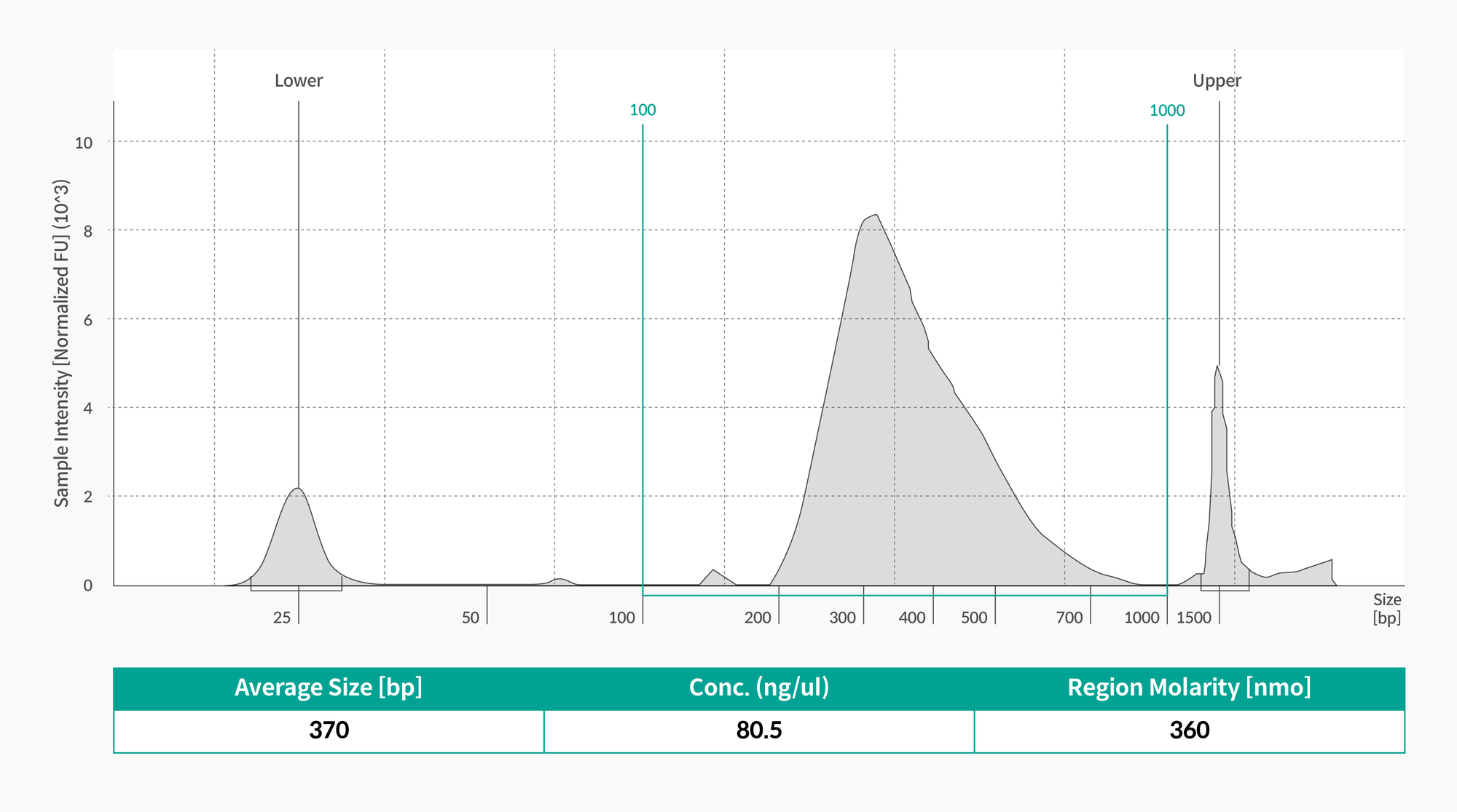
Figure 3. If the concentration is 50 ng/ul or more and the size is 350 bp and less than 430 bp, it passes library QC.
Finally, the exact quantity of the library is measured using a DNA binding dye, after which the exome capture process is carried out to select only the library components that cover exons.
Once exome capture is complete, the size and amount of the library are checked by using DNA binding dye and automated electrophoresis device.
Once the library passes all QC steps, it is now ready to be loaded into the sequencer, which once sequenced, will be the end of the long WES process. Phew!
It would not be an exaggeration to say that QC is performed at the end of every single step of the test, which may seem excessive or even annoying to some. However, as a researcher who firsthand conducts the testing, I believe the QC process is essential to maintain a high standard of reliability and obtain reproducible results.

Proband or Trio—what makes the difference?
After ensuring sequencing quality, the next step is choosing the most effective approach. See how the two compare.
Get exclusive rare disease updates
from 3billion.

Sohyun Lee
Clinical Genomics Scientist & Clinical Customer Support — guiding test selection, supporting variant and result interpretation, handling case inquiries, and translating field insights into service improvements.






