Understanding the Diagnostic Odyssey in Rare Disease Diagnosis: Journey, Challenges, and Solutions
Navigating the diagnostic journey for rare diseases can be complex and daunting for patients and their families. Often described as a “diagnostic odyssey,” this process involves extensive testing, consultations with multiple specialists, and a significant emotional toll before reaching a correct diagnosis. This article explores the intricacies of this journey, the role of advanced technologies, and the supportive measures that can improve patient outcomes.
A. The Journey to a Rare Disease Diagnosis
What is the Diagnostic Odyssey?
The term “diagnostic odyssey” refers to the protracted and challenging path many patients and families traverse to find answers about rare health conditions. Characterized by uncertainty and frustration, this journey can last several years and typically involves various healthcare providers and diagnostic tests.
Typical Pathways in the Diagnostic Journey
Symptom Onset: The patient journey typically begins when symptoms first manifest, prompting the patient to seek initial medical attention.
Visit to Primary Healthcare Provider: The first point of contact is often a primary healthcare provider who evaluates the initial symptoms.
Referrals to Multiple Specialists: Due to the uncommon and complex nature of the symptoms, the patient is usually referred to multiple specialists, each evaluating the patient from their unique medical perspective, which then leads to lacking coordination between providers.
Delayed and Misdiagnosis: The fragmented and extended nature of this pathway can lead to significant delays in reaching a definitive diagnosis.
Impact on Patient’s Well-being: The extended time to diagnosis and potential misdiagnoses compound the patient’s suffering and uncertainty, affecting their overall quality of life.

Curious how our genomic testing results look?
Get a clear view of what our reports deliver — from variant classification to clinical insight.
B. Challenges in Diagnosing Rare Diseases
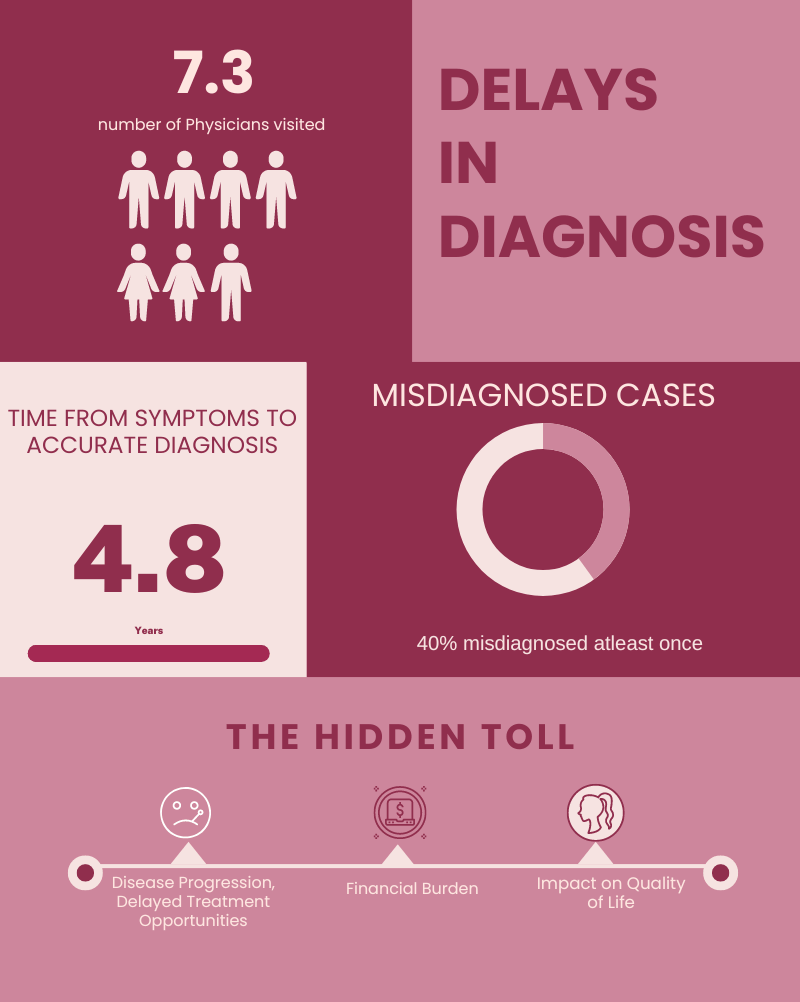
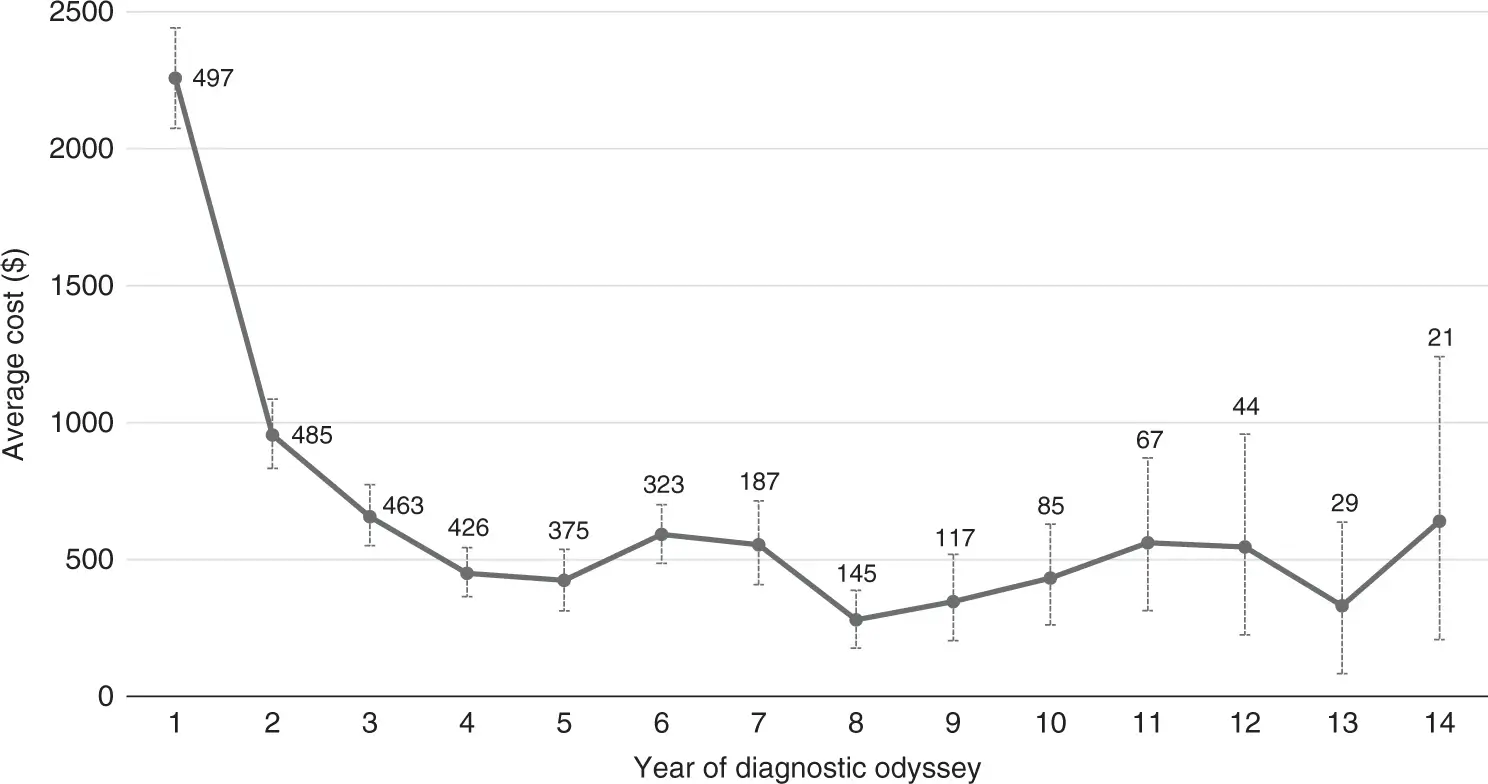
Cost, Time, and Seeking Diagnosis
In many cases, diagnoses take quite some time, especially for individuals younger than 18 years of age.
~65% of patients spend at least $4,700* across at least 6+ years before being diagnosed successfully.
~10% of patients spend at least $5,700* across 10+ years before being diagnosed successfully.
Average annual cost of ~$1,500* per family, with an average total cost of ~$5,050* before a successful diagnosis
Patients might consult with over seven specialists during this period, highlighting the need for effective care coordination and patient support.
Symptom Overlap and Misdiagnosis
One significant study highlighted that the key cause of misdiagnosis is the difficulty in accessing disease-related information. There were no disparities in misdiagnosis based on gender, age, geographical region, ethnicity, or education.
One of the significant hurdles in diagnosing rare diseases is the commonality of symptoms with more prevalent disorders. This overlap can lead to misdiagnosis, where patients receive treatment for the wrong illness, leading to ineffective outcomes and sometimes worsening the actual disease.
Ehlers-Danlos Syndrome (EDS) is an example of a rare disease that is often misdiagnosed. The symptoms of EDS can overlap with more common conditions such as fibromyalgia, chronic fatigue syndrome, and various rheumatological disorders, leading to delays in the correct diagnosis. Because the symptoms vary widely and can affect multiple body systems, EDS patients frequently undergo lengthy and complex diagnostic processes. A study found that 56% of EDS patients initially received a misdiagnosis. It took an average of 19 years for those misdiagnosed to reach the correct diagnosis compared to 8 years without a misdiagnosis.
Specialist Shortages and Geographic Barriers
The rarity of specialists in rare diseases significantly impacts patient care. Many regions lack adequately trained healthcare professionals, meaning that patients often face long waiting times for appointments. Additionally, specialists tend to be concentrated in major urban centers or academic hospitals, which may not be accessible to all, especially those in rural areas. This geographical disparity necessitates that many patients travel long distances for care, adding both physical and financial burdens to their already challenging circumstances.
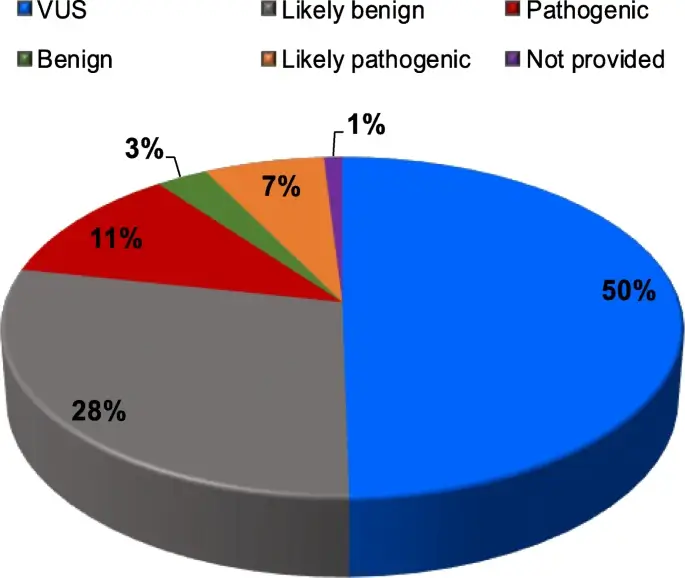
Genetic Variants and Uncertainties
One major issue is that genetic variants of uncertain significance (VUS) are often discovered through genetic testing but cannot be conclusively linked to specific conditions. This leaves both doctors and patients in a diagnostic limbo. Over time, some of these uncertainties can be resolved as more genetic data becomes available and as computational and clinical methods improve. A study from the Journal of Experimental & Clinical Cancer Research discusses the challenges and classifications of Variants of Uncertain Significance (VUS) in the context of high-throughput genome sequencing and concludes that the definition of the VUS class of variants prompted clinicians and geneticists to debate how to guarantee maximum standards for patient care.
C. How to shorten the diagnostic odyssey
Despite recommendations advocating for genetic testing to diagnose rare diseases, the utilization of such tests remains minimal, with less than 10% of individuals, including vulnerable children in intensive care units, undergoing genetic screening. Furthermore, even when genetic testing is offered, it predominantly utilizes low-resolution technologies such as chromosomal microarray or targeted gene panel tests, resulting in relatively low rates of diagnosis.
Early Genomic Testing
Based on the study, by incorporating early genomic sequencing—specifically whole-genome sequencing (WGS) and whole-exome sequencing (WES)—clinicians can significantly expedite the diagnosis process. Benefits are as follows.
Early and Accurate Diagnosis: Implementing WGS and WES at the outset of clinical assessment allows for a comprehensive analysis of an infant’s DNA, identifying potential genetic disorders swiftly and accurately.
Cost-Effectiveness: Although genomic tests are expensive, their ability to provide a quick diagnosis can lead to cost savings by avoiding a lengthy series of tests and reducing hospital stays.
Improved Patient Management: With a faster diagnosis, appropriate treatments can be administered promptly, improving the quality of life and outcomes for these young patients.
Reduction in Unnecessary Procedures: When genomic sequencing does not reveal a genetic cause, it helps to rule out genetic conditions, thus directing the diagnostic focus towards non-genetic illnesses and preventing unnecessary interventions.
Reanalysis
The genetic landscape is constantly evolving as new discoveries are made. For cases that remain unresolved after initial genomic analysis, it is crucial to periodically reanalyze existing genomic data. This can uncover new insights as the scientific community’s understanding of genetic markers and their implications grows, potentially leading to breakthroughs in many individual cases.
Integrating Genetic Databases and Interdisciplinary Communication
Creating and integrating genetic databases helps clinicians compare newly observed genetic variants with previously recorded data. This can accelerate the interpretation process and assist in identifying disease-causing mutations more quickly.
Besides this, fostering collaboration among various specialists, including geneticists, researchers, and patient advocates, can enhance the understanding and management of rare diseases. Sharing knowledge and experiences is vital in a field where many conditions are incredibly rare.
Policy and Funding Support
Advocating for policy changes that increase funding for rare disease research and improve insurance coverage for rare disease diagnosis and treatment can significantly reduce barriers to timely diagnosis.
By adopting early genomic testing, the medical community can make significant strides in reducing the diagnostic odyssey for many families, offering them clarity and the possibility of tailored treatments sooner in their journey. 3billion is your partner in navigating the genomic landscape, providing optimized genetic testing services and non-stop reanalysis to keep you at the forefront of genetic research and diagnosis. Don’t miss out on our tailored solutions—get an affordable quote today and decide at your convenience.

Not sure if this fits your needs?
Let’s explore how genomic testing can help your patients.
Get exclusive rare disease updates
from 3billion.

Sree Ramya Gunukula
Marketing Leader with experience in the pharma and healthcare sectors, specializing in digital health, genetic testing, and rare disease diagnostics.







