Overcoming Methodological Challenges in Rare Disease Research
How to Address Methodological Challenges in Rare Disease Research
Researching rare diseases is challenging due to small patient populations, diverse symptoms, and limited existing data. These challenges can make understanding patient needs and developing effective treatments difficult. This article explores strategies to overcome these hurdles, improving research outcomes and patient care.
Collaborative Research Networks
One major challenge in rare disease research is the small number of patients available for studies. Collaborative networks, like the European Reference Networks (ERNs) in Europe or similar initiatives globally, can help connect healthcare providers across countries. Collaborative networks promote the sharing of best practices and the development of clinical guidelines, ensuring high standards of care. This collaboration helps mitigate the problem of small patient populations and drives impactful research, benefiting patients worldwide.
Adaptive Trial Designs
Rare diseases often show a wide range of symptoms and progress differently in each patient. Adaptive trial designs can address this variability by allowing researchers to adjust the study based on interim results. This flexibility ensures trials are better tailored to patient needs without compromising scientific validity. By using adaptive designs, researchers can make real-time changes to the study protocol, optimizing the relevance and effectiveness of treatments.
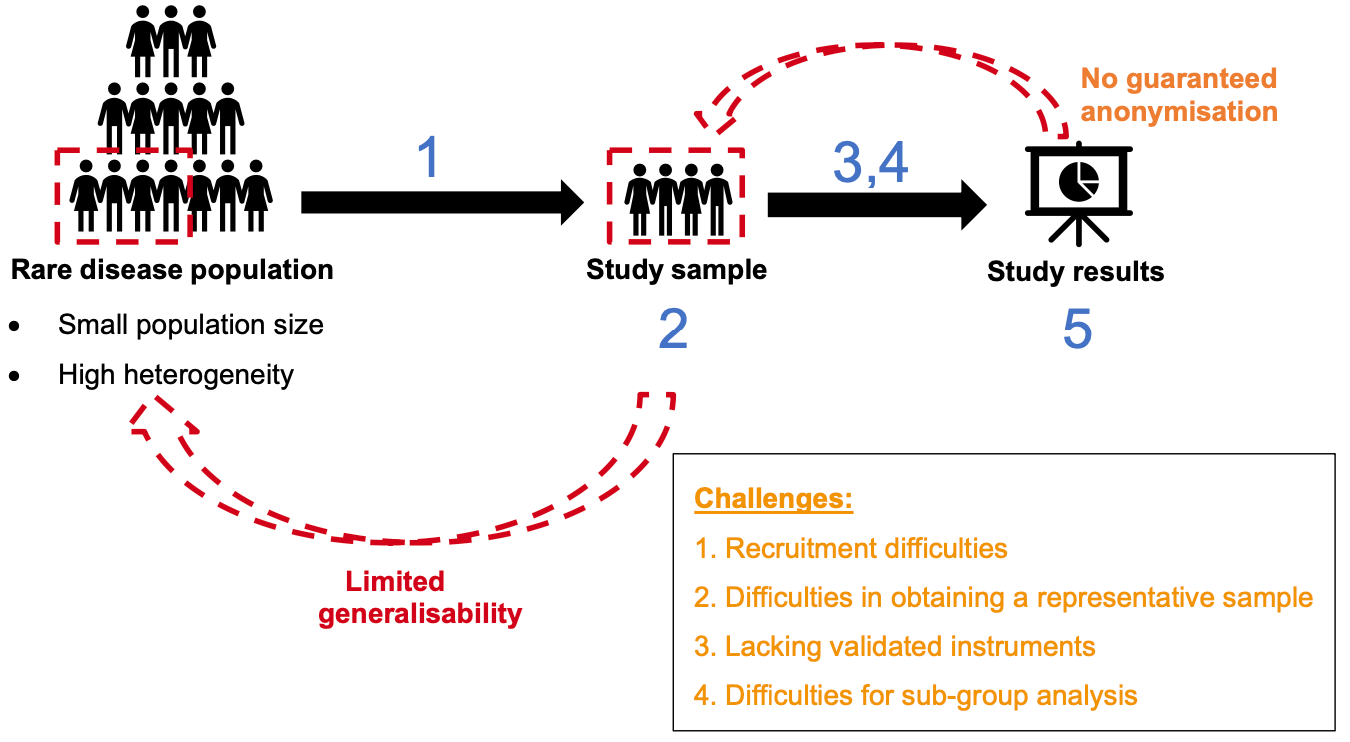
Natural History Studies and Registries
Another challenge is the lack of detailed information on how rare diseases progress over time. Conducting natural history studies and maintaining patient registries can fill this gap. These initiatives collect long-term data on disease progression, providing valuable insights for future research. Patient registries serve as databases that help identify meaningful clinical endpoints and design effective clinical trials.
Ethical Considerations in Trial Design
Ethical issues arise when conducting placebo-controlled trials in patients with no standard treatments. Using historical control data and real-world evidence can address these concerns. Historical controls provide a comparison group without exposing patients to placebos.
Harmonizing Regulatory Frameworks
Different countries have varying regulatory requirements, complicating multinational studies. Harmonizing these regulations can streamline approval processes and facilitate international collaboration. Efforts like the EU’s Orphan Regulation aim to standardize drug designation and market authorization across Europe.
Standardization and Interoperability of Data
Effective data management requires standardized methods to ensure consistency. Interoperability allows data from different sources, such as patient registries and clinical trials, to be integrated seamlessly. Developing common data elements and promoting interoperability between health information systems are key strategies. Standardized data collection improves the quality and reliability of research findings.
Innovative Funding Mechanisms
Funding limitations are a significant barrier in rare disease research. Exploring alternative funding sources like public-private partnerships, philanthropic contributions, and dedicated research grants is crucial. Programs like the EU’s Horizon 2020 provide essential financial support for innovative research and can inspire similar initiatives globally. By diversifying funding streams and fostering collaborations, researchers can secure the resources needed to advance rare disease research and develop new treatments.
Conclusion
Overcoming the challenges in rare disease research requires collaboration, innovation, and regulatory support. By leveraging networks, using adaptive trial designs, collecting detailed data, addressing ethical concerns, harmonizing regulations, standardizing data, and securing diverse funding, researchers can improve the quality and impact of their work.
Partner with us at 3billion to access advanced genetic testing solutions and expertise. Let’s collaborate to overcome research challenges and improve patient outcomes.
Contact us today to learn more!
Get exclusive rare disease updates
from 3billion.

Sree Ramya Gunukula
Marketing Leader with experience in the pharma and healthcare sectors, specializing in digital health, genetic testing, and rare disease diagnostics.







