Global rare disease diagnosis cooperation using ClinVar
What is ClinVar?
ClinVar is a public database operated by the U.S.A. National Institutes of Health (N.I.H.), in which information about variants associated with human phenotypic information is saved. Since the celebration of the submission of a million cases by 73 countries in 2019, as of Aug. 3rd, 2022, more than 2.3 million cases have been submitted by 83 countries.
The National Center for Biotechnology Information (NCBI) introduces the primary goal of operating ClinVar as below.
A major goal is to support computational (re)evaluation, both of genotypes and assertions, and to enable the ongoing evolution and development of knowledge regarding variations and associated phenotypes
Information submitted by research institutes and diagnostics companies is reviewed and registered at ClinVar, and this information includes variants and phenotypic information, and clinical significance such as Pathogenic or likely pathogenic. These are used for various studies on genetic variants ranging from cancer research to diagnosis of rare diseases.
For instance, if a doctor in South Korea shares variants and clinical information in a patient diagnosed with a specific rare disease, patients with equivalent genetic variants showing similar symptoms across the globe can be diagnosed. As such, medical institutes and diagnostics companies all over the world are cooperating to diagnose patients with rare diseases through ClinVar.
Which organizations submitted the most?
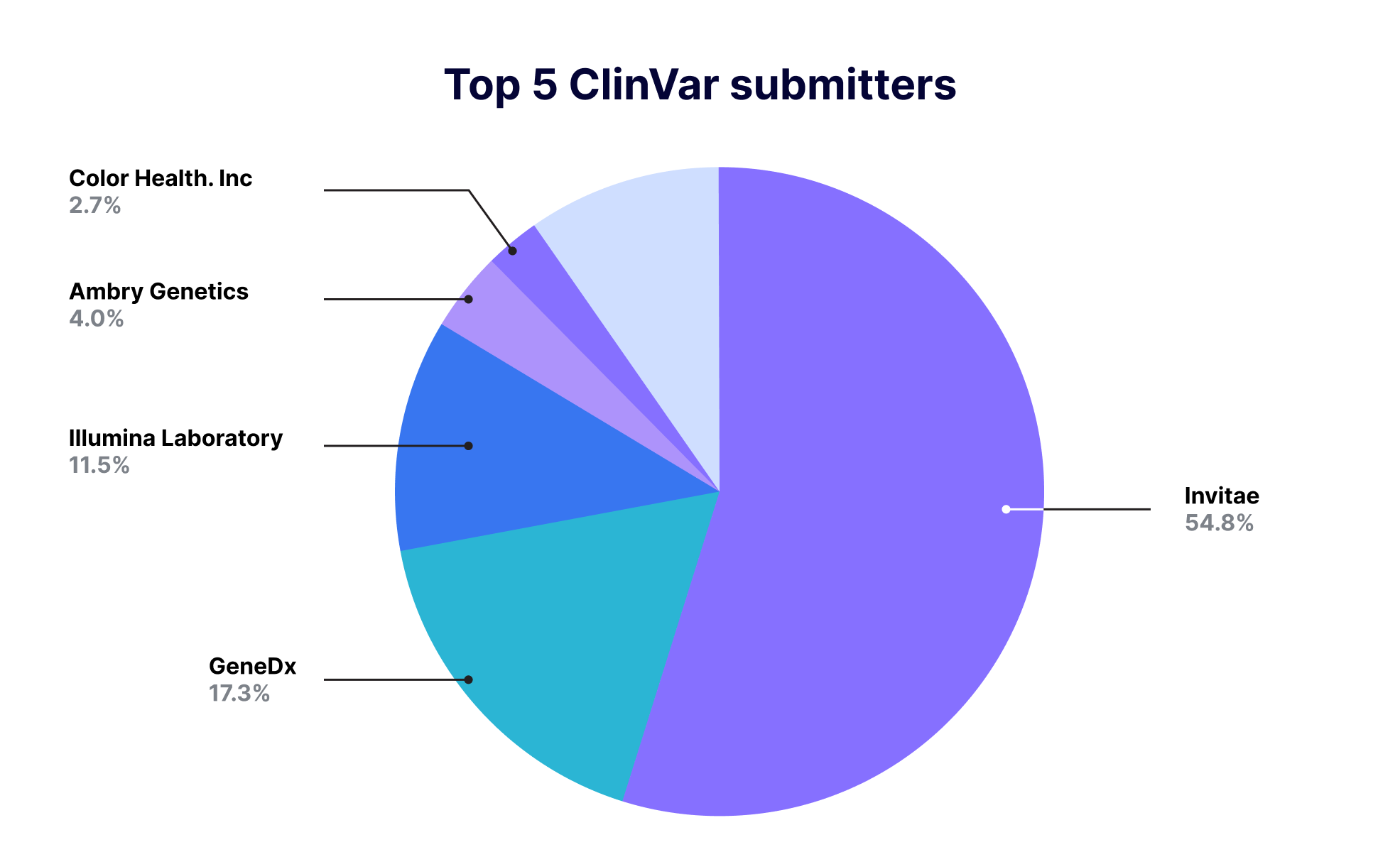
NCBI provides public access to statistical information for all data submitted to ClinVar. Looking at the data (As of August 3rd), it is found that genetic diagnostics companies as well as medical institutes proactively share information on genetic variants. In terms of number, companies share most of the genetic variant information, showing that the top 8 companies are reporting 73% of genetic mutations submitted to ClinVar, including a million cases from Invitae, 0.31 million cases from GeneDx, and 50 thousand cases from Color Health in the U.S.A.
In Asia, 30 countries reported 60 thousand cases except for Central Asia, and 3billion reported 3,074 cases, the largest number of variants reported by rare disease-specialized diagnostics companies in Asia.
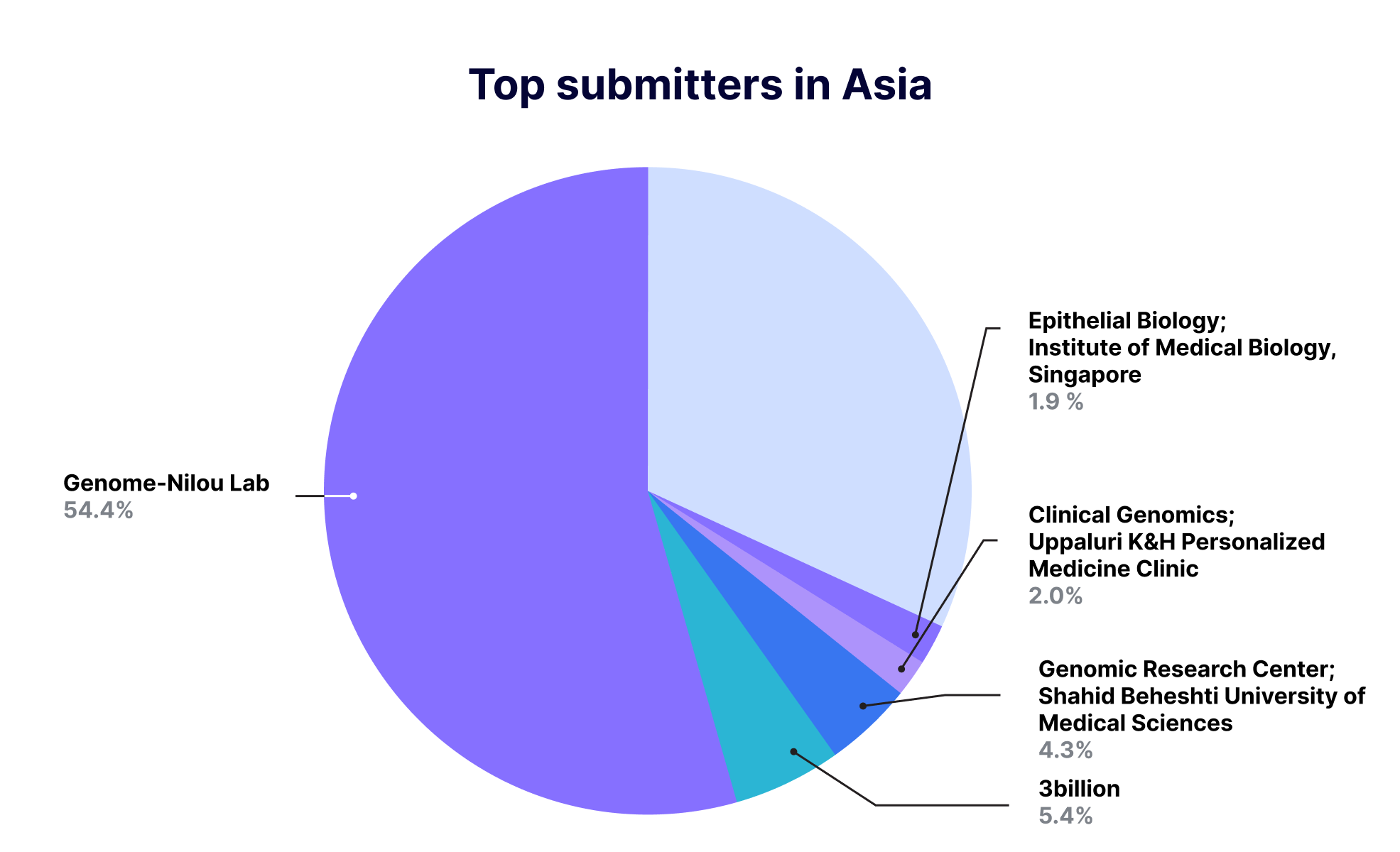
Considering that it hasn’t even been a year since 3billion started reporting on ClinVar, that’s a high number of submissions. It is expected that 3billion will be able to contribute more to the diagnosis and research of rare diseases around the world in the future.
Get exclusive rare disease updates
from 3billion.

Taeyeon Bae
Marketer & Growth hacker at 3billion. We are using a data-driven approach to improve the lives of people with rare diseases.







