Inherited Cardiovascular Disease | Genetic Testing
Although in practice guidelines from the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Rhythm Society recommend genetic testing for long QT syndrome, which causes ventricular arrhythmias, only 2.5% of patients receive it.
Genetic Testing for Inherited Cardiovascular Disease
Inherited Cardiovascular Disease is a group of genetic disorders that affect the heart and blood vessels. Despite the emphasis on importance of utilizing genetic testing for diagnose ICVD, patients with certain conditions rarely receive genetic testing recommended by clinical practice guidelines.
Why genetic testing is important for ICVD and who should be tested? Here are some important points to consider when undergoing genetic testing for ICVDs:
Case example
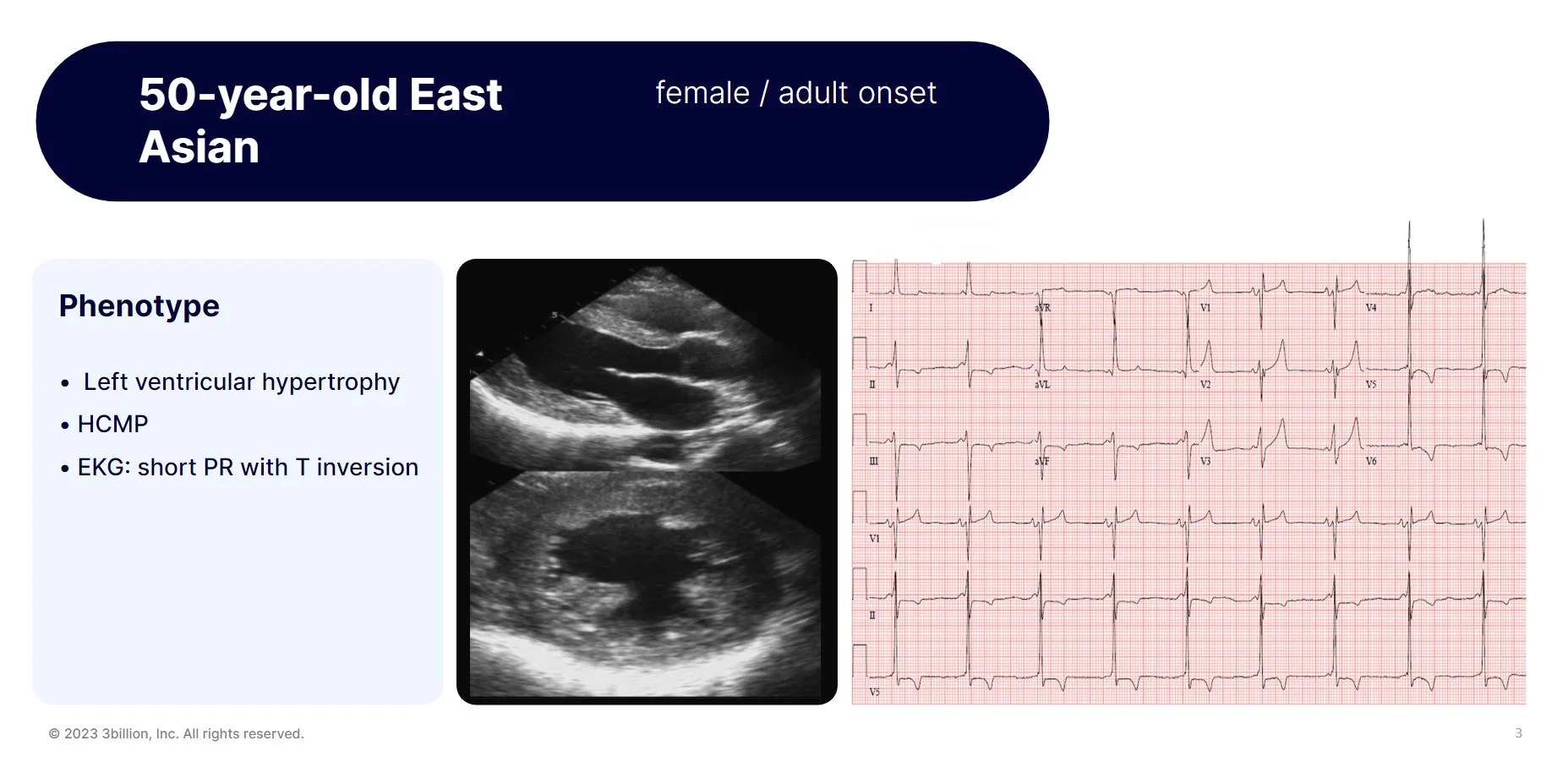
Let’s walk through a real-world case example that we worked on.
A 50-year-old East Asian female patient was reffered for exome sequencing. She had left ventricular hypertrophy, hypertrophic cardiomyopathy and short PR with T inversion on EKG as you can see in these figures. She had only received conservative treatments to relieve her symptoms. There was no previous genetic testing performed and exome sequencing was ordered as proband-only withour any other family members for testing.
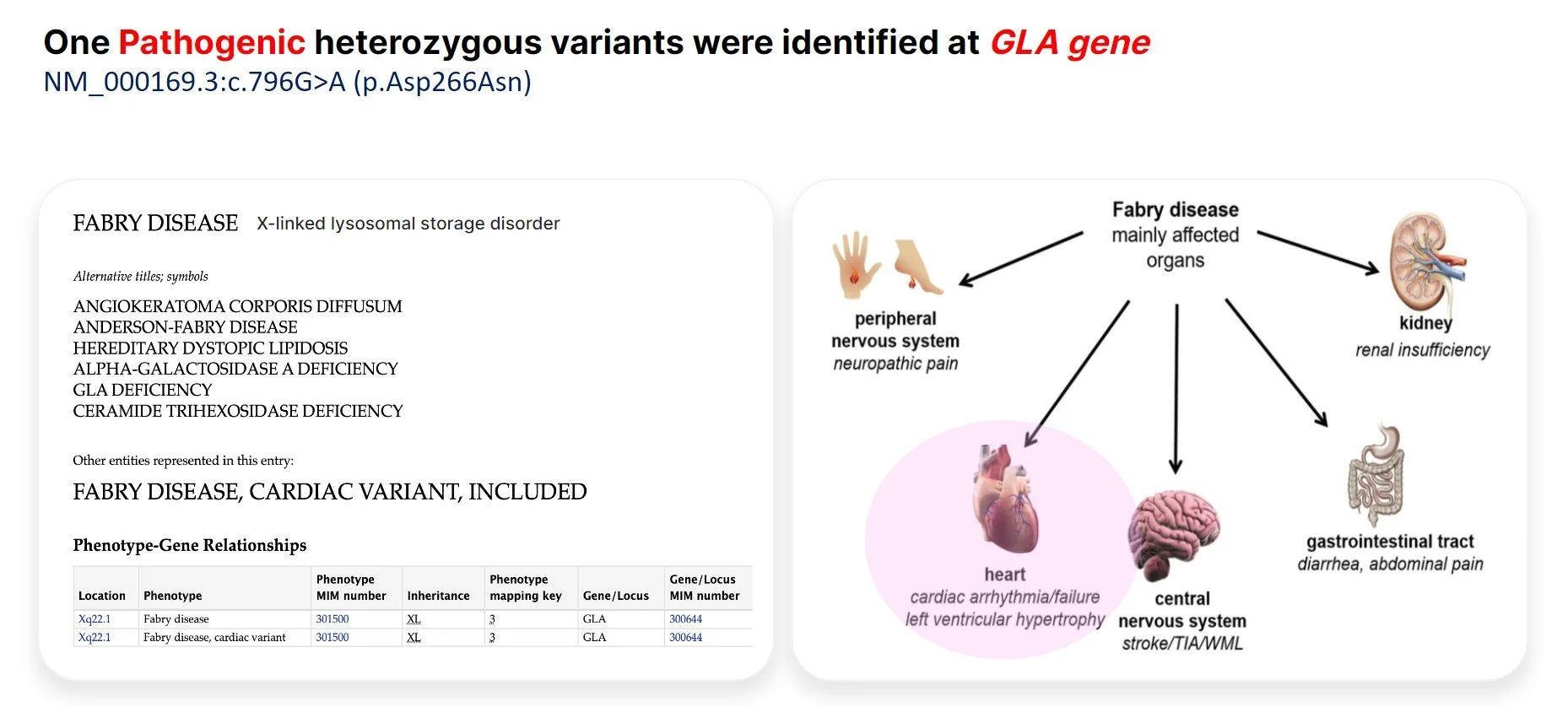
Through exome sequencing, we found a pathogenic variant in the GLA gene. The variant was a missense variant that had been reported multiple times in other patients with similar phenotype. As many of you may know, genetic defects in GLA causes Fabry disease, which is a rare X-linked lysosomal storage disease manifesting with deficiency of α-galactosidase A. The lysosomal accumulation leads to a multisystemic disease with progressive renal failure, cardiomyopathy, left ventricular hypertrophy, stroke, neuropathy, dermatologic problems, etc. as you can see in this figure, and HCMP is the most common symptom in late-onset Fabry disease.
Diagnosing the patient correctly with Fabry disease is extremely important because there are several treatments available including enzyme replacement therapy, small molecule drugs, and gene therapy. In particular, enzyme replacement therapy, the most commonly used treatment, has shown to improve symptoms, slow disease progression, and prevent complications.
So, this patient was diagnosed with Fabry disease and eventually started receiving the enzyme replacement therapy. As family screening can help identify family members at risk and allow for earlier diagnosis and treatment, genetic testing was performed on the patient’s son, mother, sister and sister’s daughter, all of whom were found to have the same GLA variant. The patient’s son, mother and sister were able to receive the enzyme replacement therapy in time as they began to show symptoms.
Utilities of Genetic Testing for Cardiovascular Disease
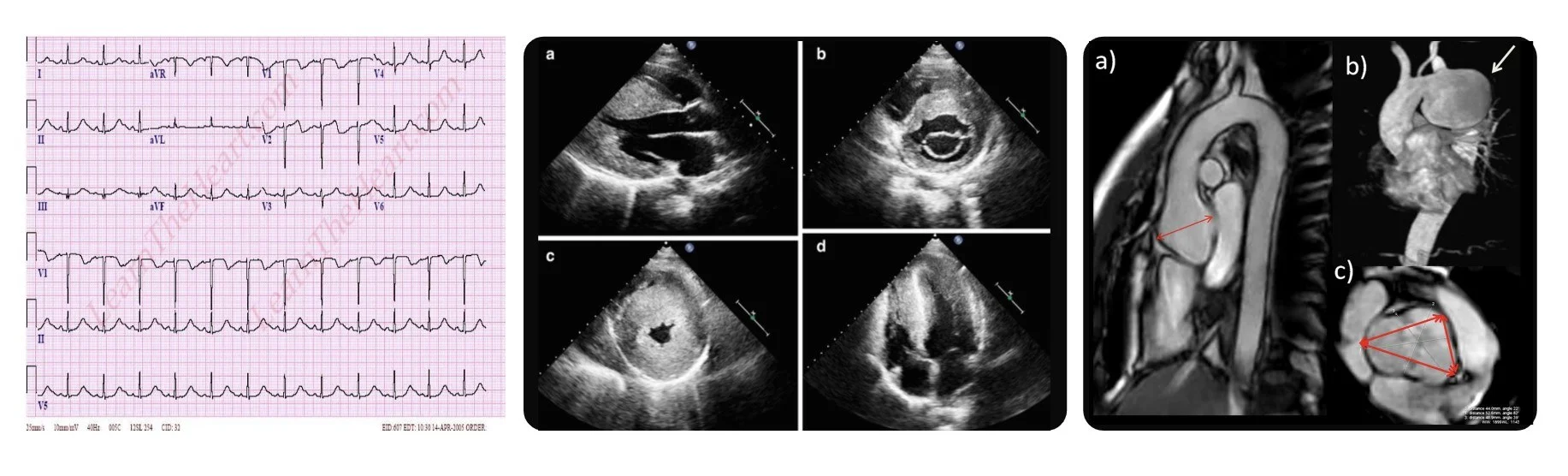
You should all know what these figures are showing. EKGs and imaging such as echocardiogram, CT and MRI definitely provide important information about patient’s current cardiac condition and help with the diagnosis and managements.
However, as shown in the case example just shared, if we knew exactly what disorder the patient has, a whole different level of managements may suddenly become available. Let’s get into more details:
Exact diagnosis
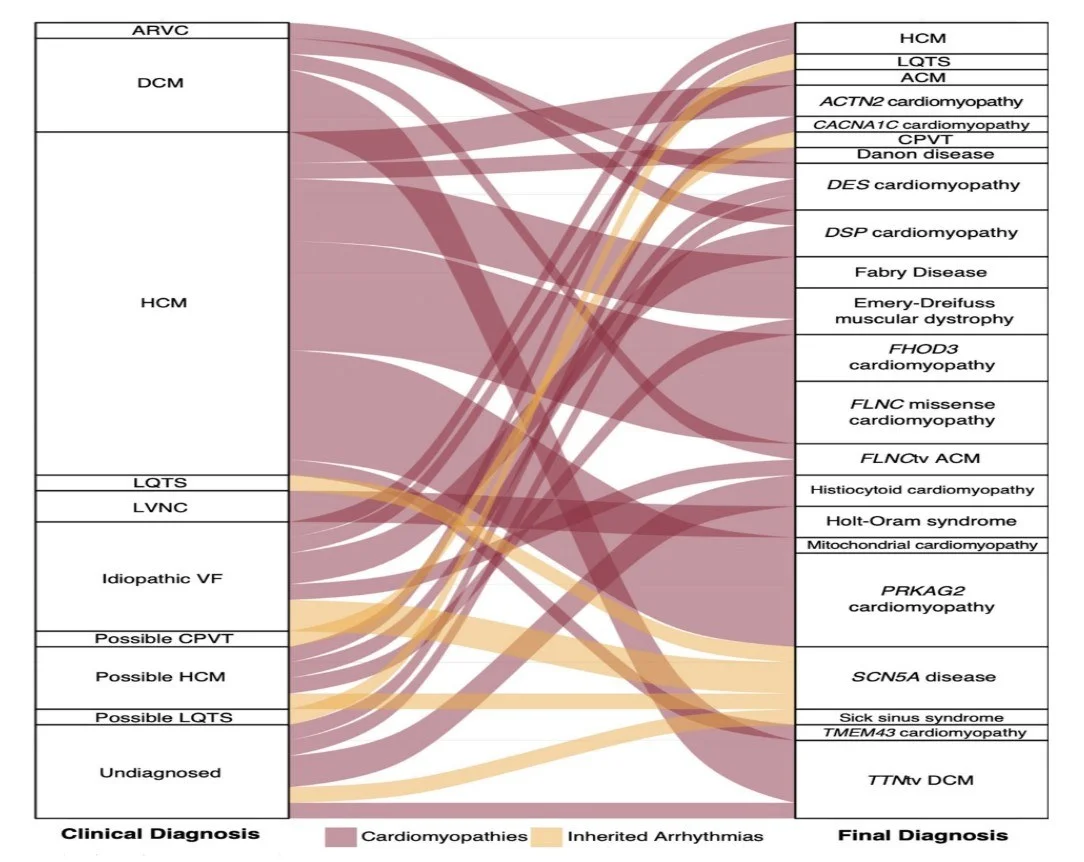
This figure shows how genetic diagnosis can clarify what patients actually have.
For example, there are a lot patients with hypertrophic cardiomyopathy but some of those patients have Fabry disease as you’ve seen in the example and others have defect in sarcomeric proteins. Why is knowing the exact underlying condition important?
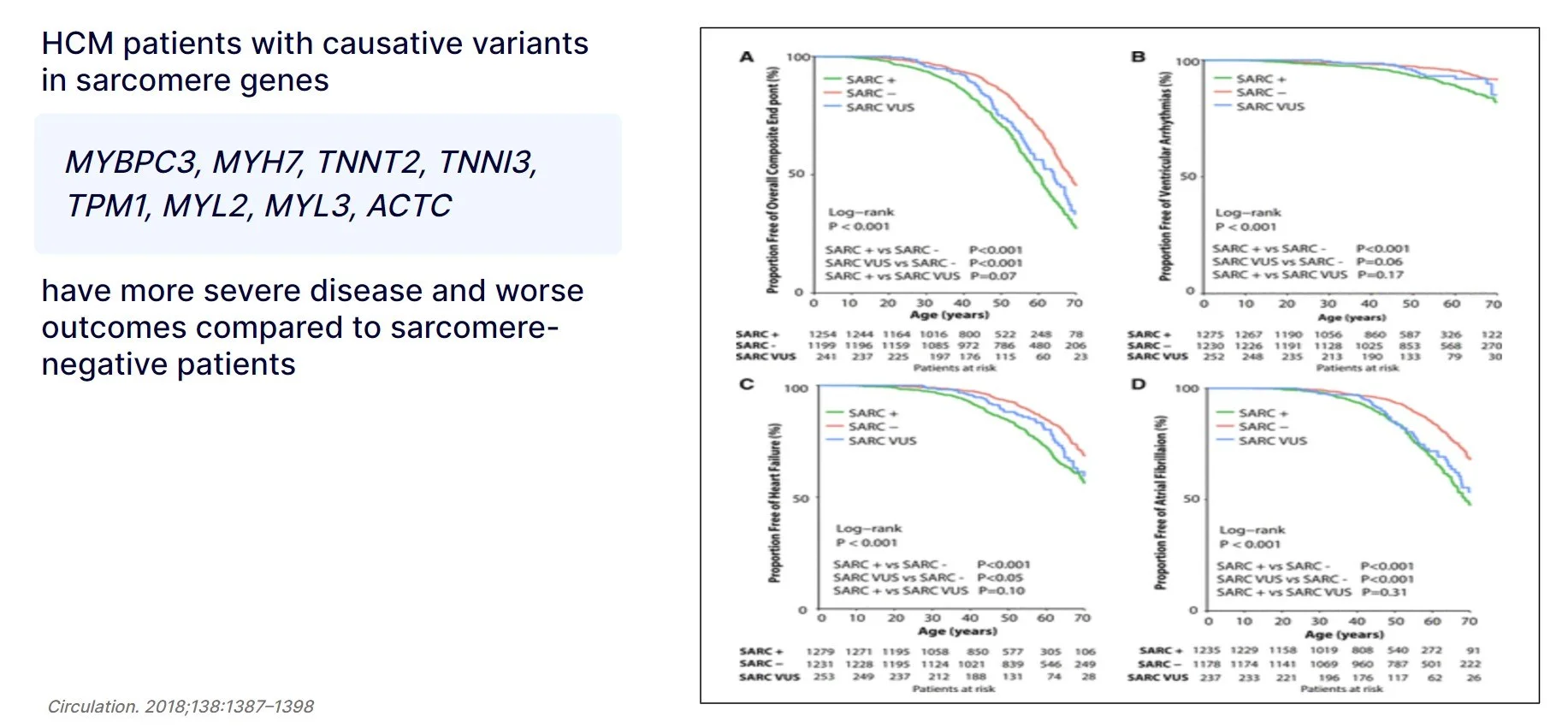
Survival analyzes shows that patients with pathogenic variants in the sarcomere genes listed here had a significantly earlier onset of the disease and a higher incidence of heart failure, ventricular arrhythmia, and atrial fibrillation than patients without a variant in these genes. This suggests that HCM patients with genetic defect in these genes need to be more closely monitored.
Family screening
The second reason why genetic testing is important is family screening.
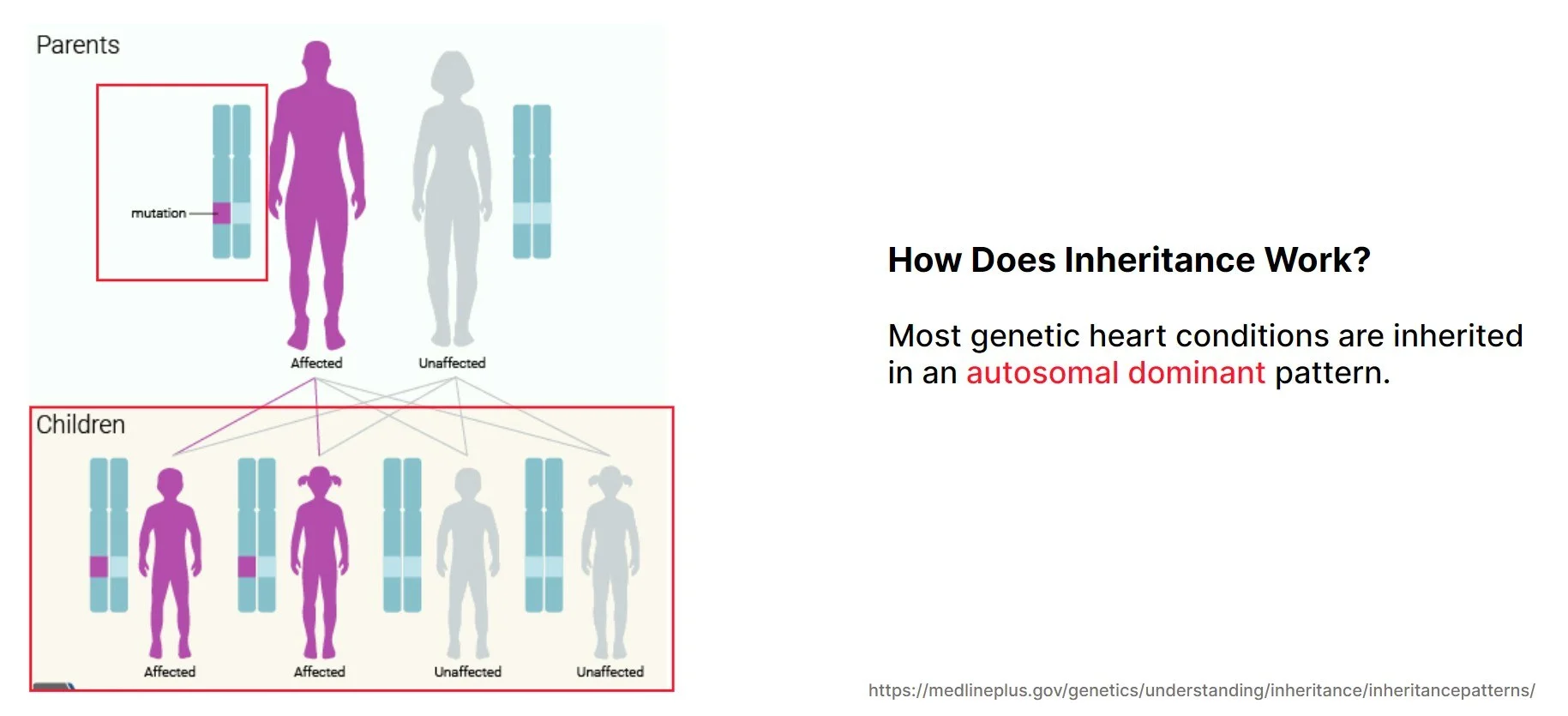
Most genetic heart diseases are inherited in an autosomal dominant pattern. For those who are not familiar with genetic terminologies, autosomal means chromosomes 1 to 22 and not the sex chromosomes so both males and females are equally affected. Dominant means that 1 defective copy of the gene is enough to cause the disease.
For autosomal dominant disease, there is a 50% chance that the abnormal copy of the gene will be passed on to a child. So, each child has a 50% chance of inheriting the abnormal copy and a risk of developing the same condition that the affected parent has. As a unit of a family, approximately half of the progeny is expected to develop the disease.
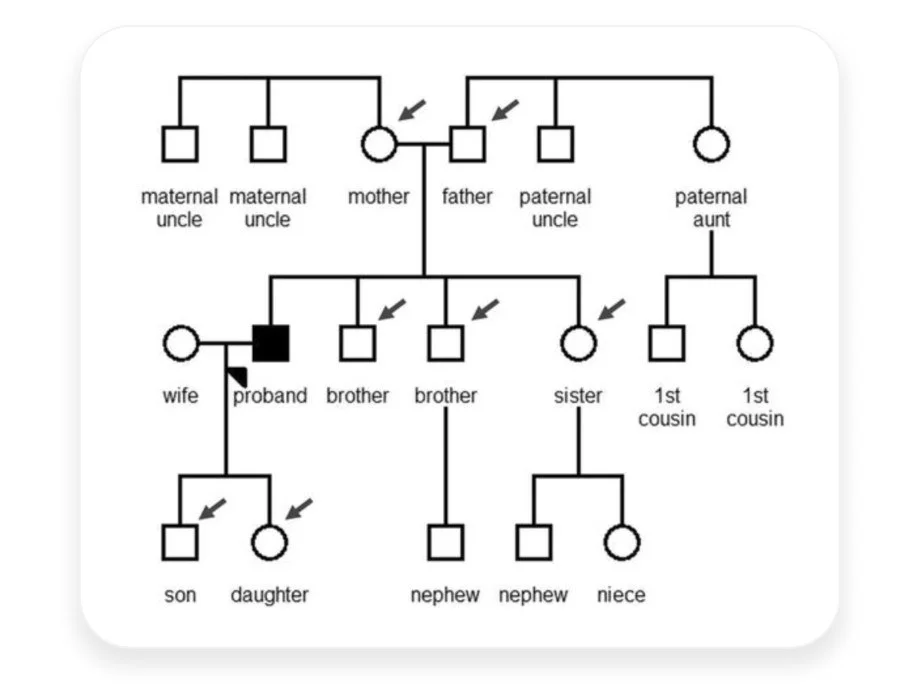
So the implication of genetic testing expands beyond the index patient to familial members at risk.
As you can see in these figures, nearly half of the family members received a positive genetic test results as expected for an autosomal dominant disease. Positive results are medically actionable and enable continuous monitoring, and negative results allow family members to avoid intensive longitudinal screening and reduce medical costs.
In summary, genetic testing can lead to accurate diagnosis followed by appropriate clinical management, and screening of at-risk family members to prevent disease progression and avoid excessive healthcare cost.
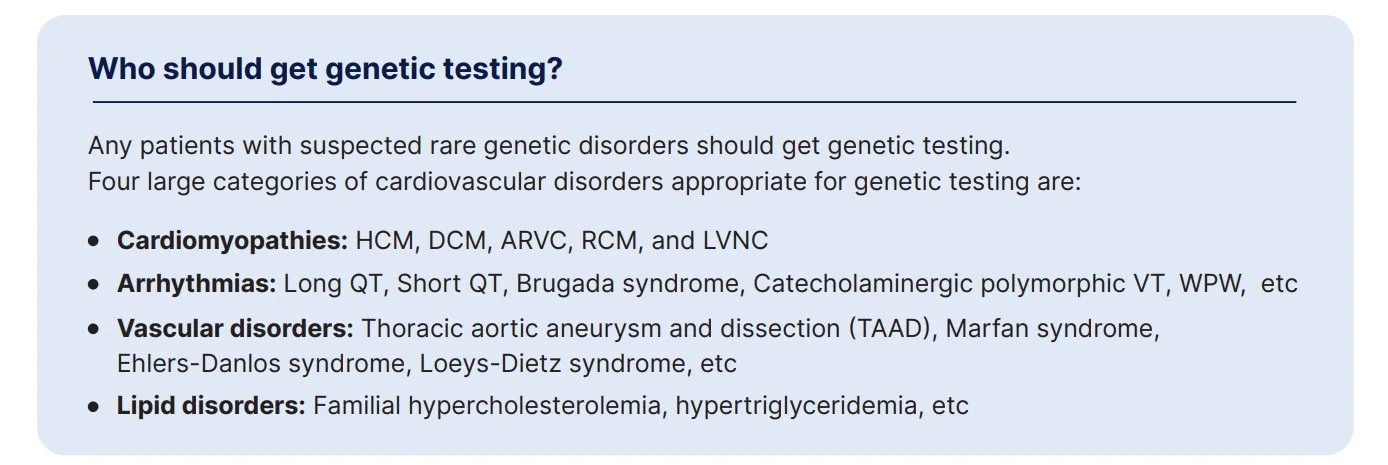
Who should get genetic testing?
Genetic testing is informative for a variety of inherited cardiovascular diseases that can be grouped into these four categories: cardiomyopathies including HCMP, DCMP, ARVC, RCM, and LVNC, Arrhythmias, vascular disorders such as Thoracic aortic aneurysm and dissection, and lipid disorders.
The American Heart Association Scientific Statement summarizes current best practices for patients with confirmed or suspected genetic disorders.
What test options are available?
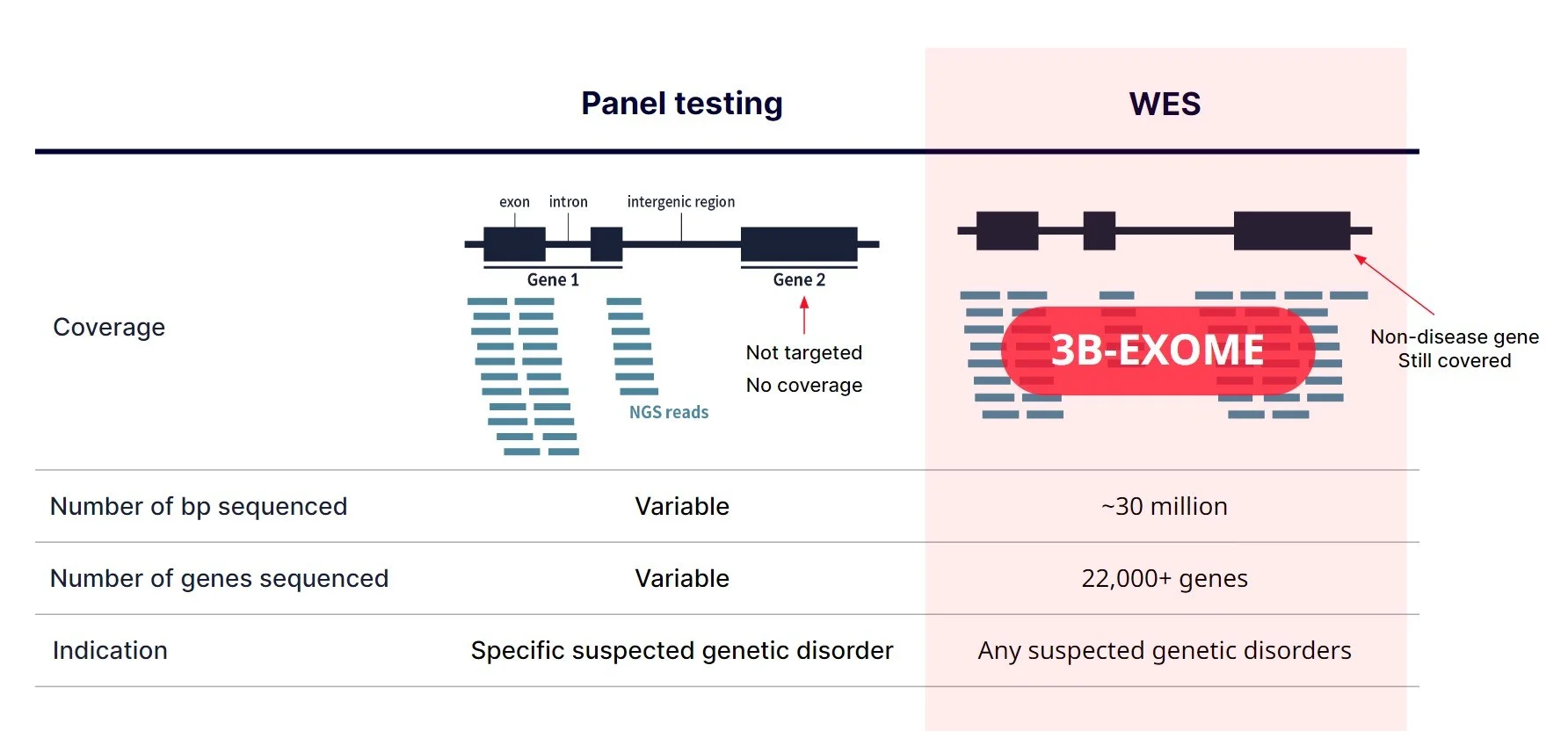
Traditionally, each gene was individually sequenced, or a small gene panel was done using sanger sequencing.
With the advent of NGS, however, it became possible to sequence many genes simultaneously, so laboratories started to create larger gene panels for each disorder. The size of the panels vary but basically, the targeted genes on the panel will be sequenced as you can see on the left. Most of the panels only target the coding exons.
When physicians decide to order a gene panel, a specific panel or panels are selected based on the patient’s symptoms. For instance, if the patient has hearing loss, a hearing loss panel is ordered.
On the other hand, whole exome sequencing targets the protein coding exons of almost all genes. Since a majority of the known pathogenic variants are within the exonic regions, it is a cost-effective way to identify disease causing variants when a large number of genes need to be tested simultaneously or when it’s difficult to specify genes that need to be tested. Pretty much any patient with suspected genetic disorder can be tested on exome.
Then, for the cardiovascular disorders, which is more appropriate to use, panel or exome?
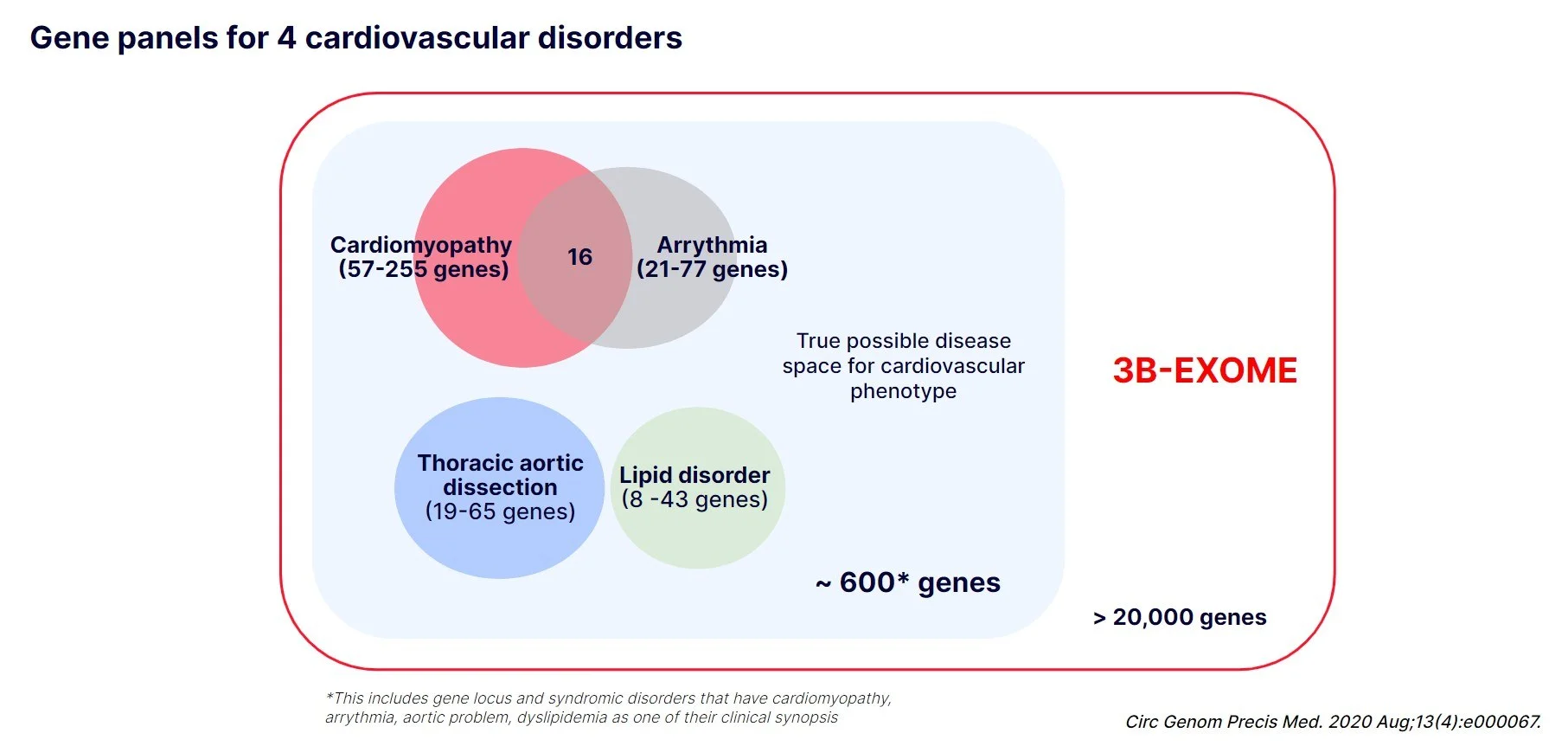
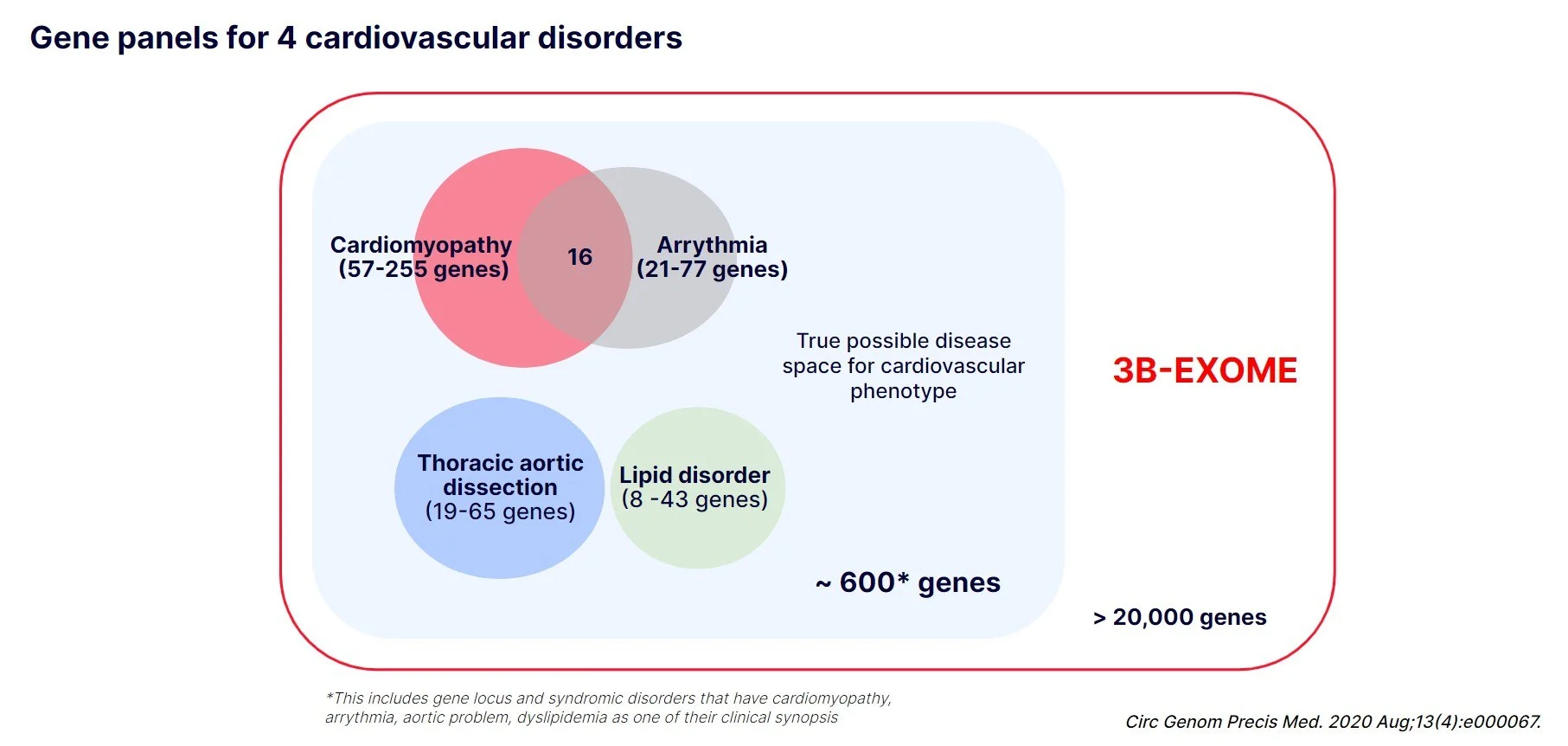
Here we’re showing the size and overlap of the 4 commonly used gene panels. Different laboratories could have different sets of genes on each panel so we collected information from three large reference labs and panelapp from genomics england.
In total, approximately 450 genes are included in these four panels with a few genes overlapping between cardiomyopathy panel and arrhythmia panel.
However, when we search omim for these 4 phenotypes, over 600 genes come up, meaning that there are genes not included in the panels that could manifest one or more of these 4 symptoms. Many of these genes that are not on the panel are mostly because they cause syndromic disorders, but it is possible that a patient might have only presented cardiomyopathy at the time of testing or was overlooked for other symptoms.
If you run exome as a first-tier testing though, you would sequence all 600 genes plus the rest of the genes that are not yet a disease gene.
What you need to know more about exome sequencing
Diagnostic Yield
Pretty much the only reason is that exome price is slightly higher than panel price and that, in some countries, it is easier to have insurance cover panels than exomes. However, overall, more patients would be diagnosed by exomes than by panels. Let’s quickly review the diagnostic rates for cardiovascular disorders.
According to the recent study, around 20% of patients with cardiomyopathy or arrhythmia panel testing have a positive genetic test result.
To compare with, we also searched our internal database to find 2444 adult patients with cardiovascular symptoms who were referred for exome.
The most common symptoms were left ventricular hypertrophy, followed by congestive heart failure, and arrhythmia as shown in word cloud on the left.
In total, 26% of these patients received a positive result with 69 different genes and the largest group, cardiomyopathy and arrhythmia also had the diagnostic rate of 26%. Vascular disorder group had a 21% diagnostic rate and lipid disorder group had a 25% diagnostic rate. However, as the Ns were small and the 95% confidence intervals are wide.
It means that 6% of patients could not have been diagnosed if a panel were performed instead of exome as the genes are not on any of the commercial panels.
Do not hesitate!
Wish this article could convince you that genetic testing for cardiovascular patients is useful and have you re-think with us how clinical practice could change by integrating genetic testing at an earlier stage of diagnosing patients.
By implementing genomic sequencing as a first-line diagnostic tool, the time and cost for precise diagnosis will be reduced and patients will be able to receive more personalized clinical management.
Get exclusive rare disease updates
from 3billion.

YoonJung Shin
Content Marketer