5 reasons patients with CA/DD/ID should get whole exome/genome sequencing tests
In 2021, the American College of Medical Genetics and Genomics (ACMG) suggested the following: “We strongly recommend that ES/GS be considered as a first- or second-tier test for patients with CA/DD/ID”, where ES/GS means exome sequencing and genome sequencing, respectively. Here, CA, DD, ID means congenital anomalies, developmental delay, and intellectual disability, respectively.
There are two main types of genetic tests: standard tests are faster and cheaper but test only a small number of genes and variants. ES/GS is slower and more expensive but tests almost all variants in the exome/genome.
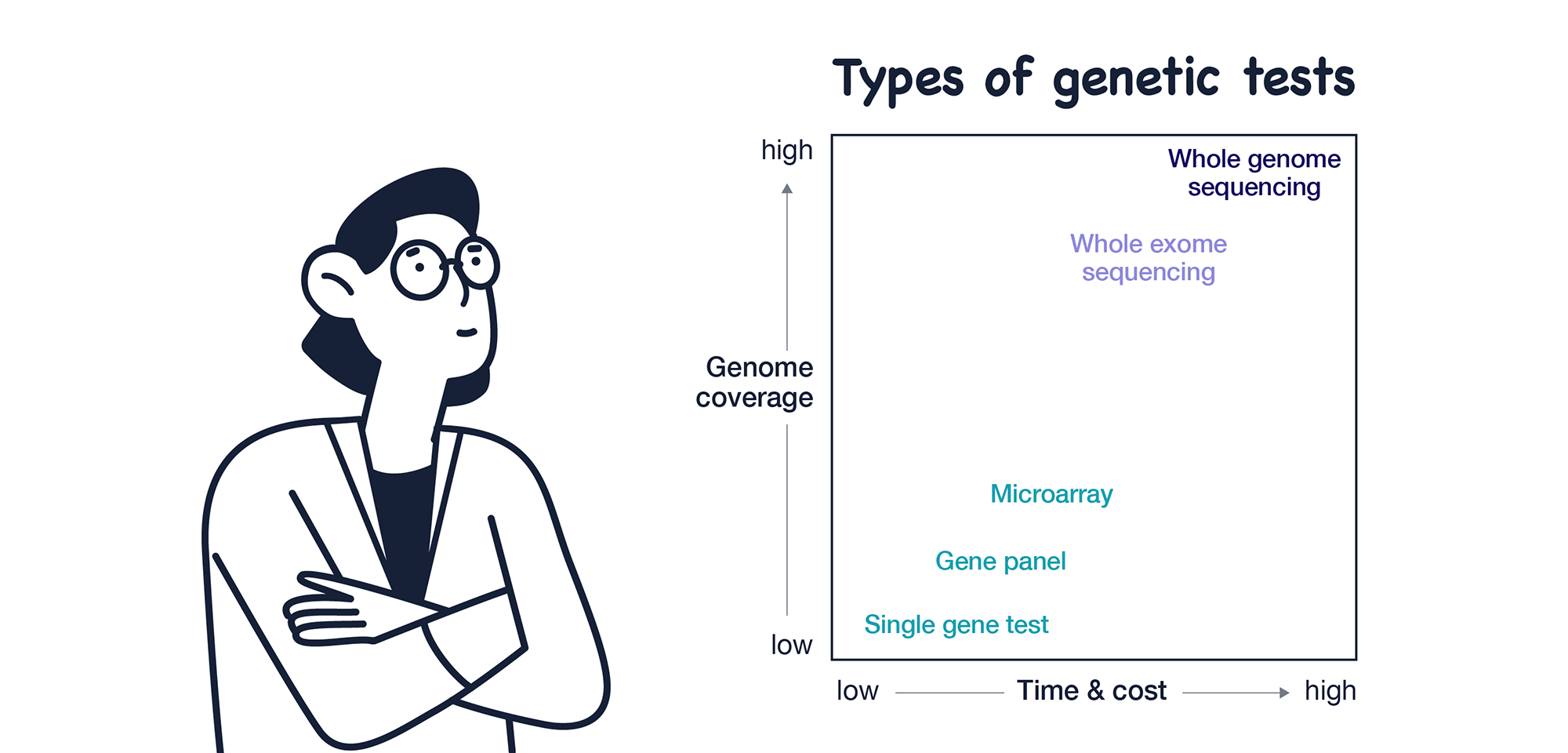
Then why does ACMG suggest ES/GS tests? Let’s look at five main reasons they suggest ES/GS tests.
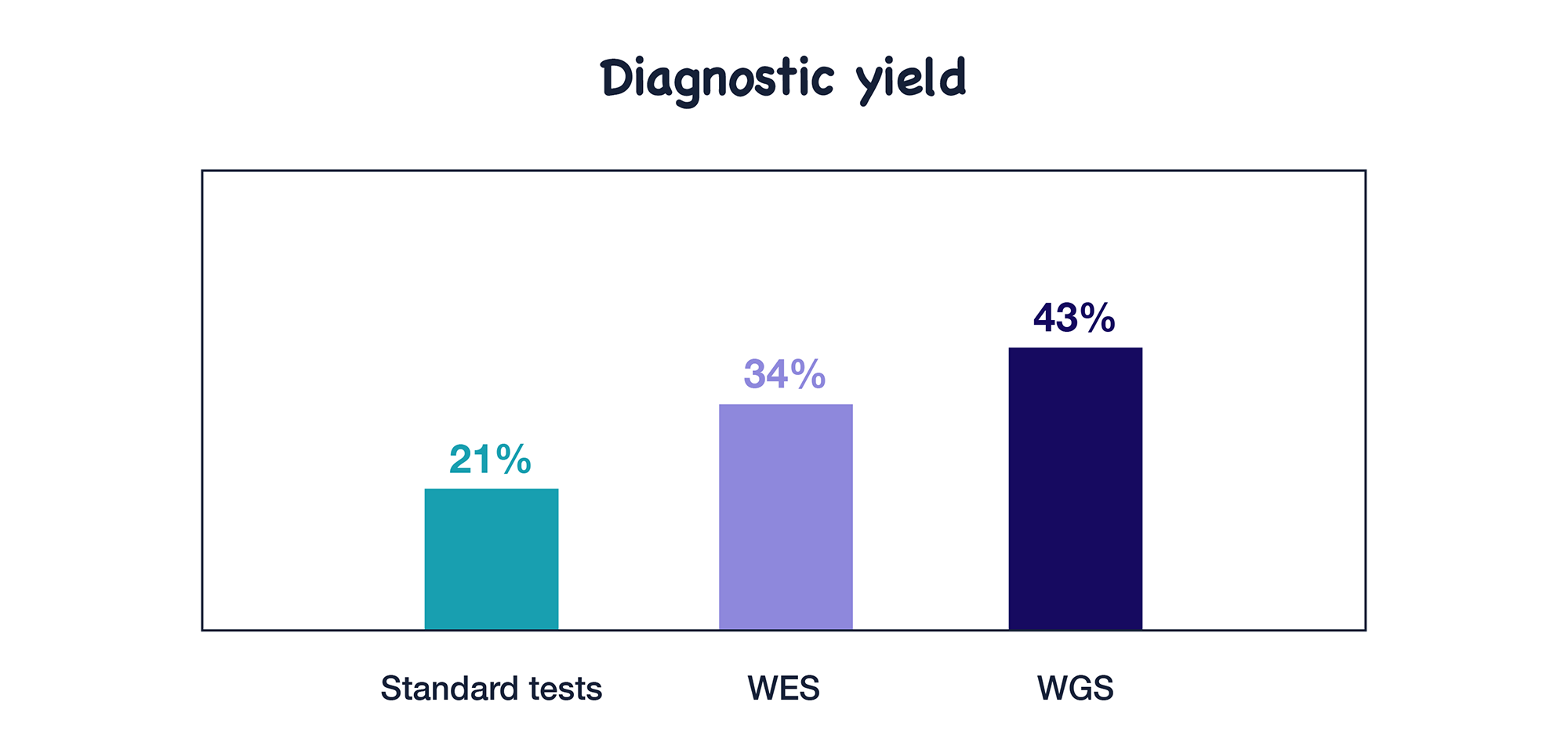
1. Diagnostic yield
Although diagnostic yield is not the focus of this guideline, it is one of the most important aspects for patients and their families. After reviewing studies comparing diagnosis with standard tests versus ES/GS, ACMG found that ES/GS showed higher diagnostic yield than standard tests.

Access Our Webinars on WGS and WES
Watch our expert-led sessions on how WGS and WES are revolutionizing the diagnosis of rare diseases.
2. Cost-effectiveness
According to the Ontario Health Technology Assessment (HTA), using ES/GS as a first- or second-tier test was found to yield more diagnoses at a lower cost than using standard tests first. Also, the sequencing cost is anticipated to continuously decrease.
3. Clinical management
A genetic test can change the course of patients’ clinical management. ACMG reviewed 25 studies and found that ES/GS impacted short-term clinical management of 8% of patients and long-term clinical management of 10% of patients.
4. Undesirable effects
ACMG also checked for any potential harms of ES/GS tests. These include discrimination in insurance coverage, negative impact on family dynamics or communication, financial burden of the costs associated with additional testing, surveillance, medication, or dietary modifications stemming from the results of ES/GS, general negative psychosocial impact to the patient or their family, and reduction or loss of privacy.
Among the 167 studies, ACMG found few negative impacts due to ES/GS results. They could not find clinically significant psychological harms from the ES/GS results. Some cases showed low levels of test-related distress or greater positive psychological effects.
5. Value
Patients and their families obtained motivation and benefits from diagnosis for unexplained developmental diseases and congenital anomalies. Also, they greatly valued the support and the information provided through genetic counseling when they considered ES/GS and learned about the diagnosis.
Conclusion
After a thorough review of many clinical studies, ACMG strongly recommended ES/GS as a first- or second-tier test for CA/DD/ID patients. With little evidence of negative effects and growing evidence of benefits, ES/GS has proven to be useful both clinically and for the patient’s family.
👉 In this article, we discussed why genomic testing such as WES/WGS should be considered for patients with global developmental delay (GDD) or intellectual disability (ID).
Since then, the American Academy of Pediatrics (AAP) has published its 2025 clinical guideline, recommending whole exome or genome sequencing as a first-tier test for such cases.
Learn more about this shift in clinical diagnostic strategy in our follow-up post:
➤ [Read the follow-up] Which genetic test should come first for a child with developmental delay?
🧬 Wondering if a suspected condition can be diagnosed via WES or WGS?
→ Check the Gene Coverage Browser to explore coverage details for specific genes.
Reference
Manickam, K., McClain, M.R., Demmer, L.A. et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genetics in Medicine. 2021.
Get exclusive rare disease updates
from 3billion.

Heonjong Han
Lead manager in bioinformatics team, responsible for development of variant interpretation program, EVIDENCE.







