ACMG Secondary Findings v3.3 Released
Understanding ACMG Secondary Findings: A Deep Dive into the Updated 84 Genes and Why Patient Consent Matters
What Are Secondary Findings?
In genetics, secondary findings refer to results unrelated to the primary reason for ordering a genetic test. It’s akin to discovering a suspicious mass during an X-ray for a fractured bone—an incidental yet potentially significant finding.
Traditionally, genetic testing focused only on genes suspected of being related to a patient’s symptoms. But with the rise of whole exome sequencing (WES) and whole genome sequencing (WGS), we now analyze a much broader range of genes—providing powerful diagnostic value, especially for patients with complex or unclear symptoms.
However, this comprehensive approach increases the chance of identifying pathogenic variants unrelated to the patient’s current health issues. When these variants occur in genes associated with serious but manageable conditions, the findings may allow for prevention, early diagnosis, or timely treatment.
These are called secondary findings.
Which Genes Are Included?
The updated v3.3 list released by the American College of Medical Genetics and Genomics (ACMG) in June 2025 includes 84 genes associated with hereditary cancer syndromes, cardiovascular diseases, and metabolic disorders.
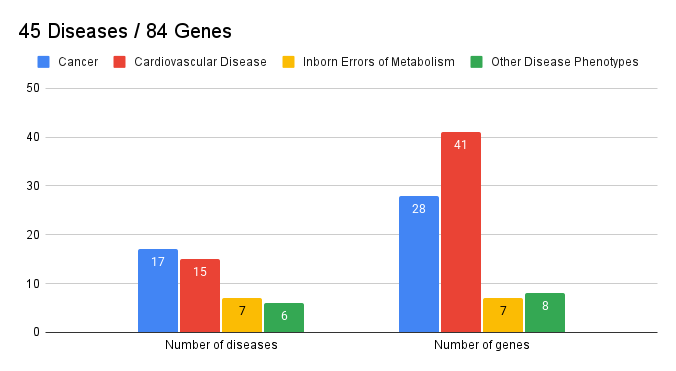
These genes were selected because identifying pathogenic variants in them can lead to a change in medical management—enabling early intervention that can potentially prevent disease or improve outcomes.
Importantly, only variants classified as pathogenic or likely pathogenic, and clinically actionable based on the mode of inheritance, are reported. The mere presence of a variant in these genes does not automatically qualify as a secondary finding.
Who Defines These Genes?
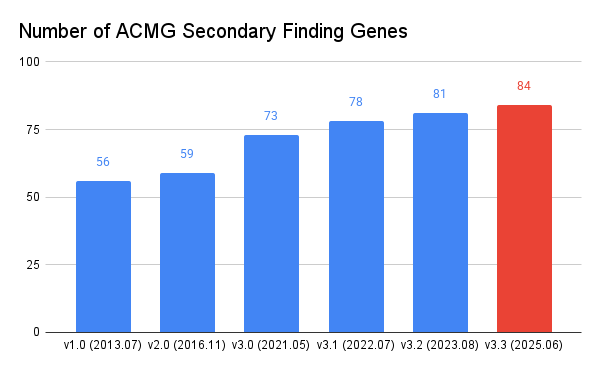
The ACMG is a global authority that develops standards and guidelines in clinical genetics. It first introduced the concept of secondary findings in 2013. Since then, the list has evolved based on new evidence and clinical relevance, culminating in the current version (v3.3) with 84 genes.
Why Is Informed Consent Essential?
While secondary findings can offer life-saving insights, they also come with ethical and psychological considerations.
For some, learning about a high-risk condition they weren’t expecting—or may never develop—can be emotionally distressing. It may lead to additional testing, long-term monitoring, or healthcare costs. In the case of pediatric patients, such findings might reveal health risks before the child is old enough to make an informed decision.
That’s why patients and their families are always given a choice:
They can opt out of receiving secondary findings altogether. This consent process is a core component of ethical genomic testing.
If you’d like to explore the full ACMG v3.3 list or understand how secondary findings may apply to your clinical practice, stay tuned—we’ll be sharing more resources soon.
Get exclusive rare disease updates
from 3billion.

3billion Inc.
3billion is dedicated to creating a world where patients with rare diseases are not neglected in diagnosis and treatment.






